当前位置:
X-MOL 学术
›
Cancer Treat. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Current standards and future perspectives in adjuvant treatment for biliary tract cancers.
Cancer Treatment Reviews ( IF 9.6 ) Pub Date : 2019-12-04 , DOI: 10.1016/j.ctrv.2019.101936 Angela Lamarca 1 , Julien Edeline 2 , Mairéad G McNamara 1 , Richard A Hubner 1 , Masato Nagino 3 , John Bridgewater 4 , John Primrose 5 , Juan W Valle 1
Cancer Treatment Reviews ( IF 9.6 ) Pub Date : 2019-12-04 , DOI: 10.1016/j.ctrv.2019.101936 Angela Lamarca 1 , Julien Edeline 2 , Mairéad G McNamara 1 , Richard A Hubner 1 , Masato Nagino 3 , John Bridgewater 4 , John Primrose 5 , Juan W Valle 1
Affiliation
|
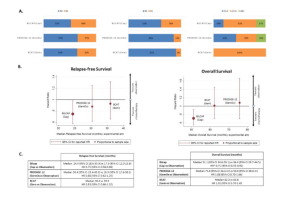
Biliary tract cancer, including cholangiocarcinoma (CCA) and gallbladder cancer (GBC) are rare tumours with a rising incidence. Prognosis is poor, since most patients are diagnosed with advanced disease. Only ~20% of patients are diagnosed with early-stage disease, suitable for curative surgery. Despite surgery performed with potentially-curative intent, relapse rates are high, with around 60-70% of patients expected to have disease recurrence. Most relapses occur in the form of distant metastases, with a predominance of liver spread. In view of high tumour recurrence, adjuvant strategies have been explored for many years, in the form of radiotherapy, chemo-radiotherapy and chemotherapy. Historically, few randomised trials were available, which included a variety of additional tumours (e.g. pancreatic and ampullary tumours); most evidence relied on phase II and retrospective studies, with no high-quality evidence available to define the real benefit derived from adjuvant strategies. Since 2017, three randomised phase III clinical trials have been reported; all recruited patients with resected biliary tract cancer (CCA and GBC) who were randomised to observation alone, or chemotherapy in the form of gemcitabine (BCAT study; included patients diagnosed with extrahepatic CCA only), gemcitabine and oxaliplatin (PRODIGE-12/ACCORD-18; included patients diagnosed with CCA and GBC) or capecitabine (BILCAP; included patients diagnosed with CCA and GBC). While gemcitabine-based chemotherapy failed to show an impact on patient outcome (relapse-free survival (RFS) or overall survival (OS)), the BILCAP study showed a benefit from adjuvant capecitabine in terms of OS (pre-planned sensitivity analysis in the intention-to-treat population and in the per-protocol analysis), with confirmed benefit in terms of RFS. Based on the BILCAP trial, international guidelines recommend adjuvant capecitabine for a period of six months following potentially curative resection of CCA as the current standard of care for resected CCA and GBC. However, BILCAP failed to show OS benefit in the intention-to-treat (non-sensitivity analysis) population (primary end-point), and this finding, as well as some inconsistencies between studies has been criticised and has led to confusion in the biliary tract cancer medical community. This review summarises the adjuvant field in biliary tract cancer, with evidence before and after 2017, and comparison between the latest randomised phase III studies. Potential explanations are presented for differential findings, and future steps are explored.
中文翻译:

胆道癌辅助治疗的当前标准和未来前景。
胆道癌,包括胆管癌(CCA)和胆囊癌(GBC)是罕见肿瘤,且发病率不断上升。预后很差,因为大多数患者被诊断为晚期疾病。只有约 20% 的患者被诊断为早期疾病,适合进行根治性手术。尽管手术具有潜在的治愈目的,但复发率很高,预计大约 60-70% 的患者会出现疾病复发。大多数复发以远处转移的形式发生,其中以肝脏扩散为主。鉴于肿瘤的高复发率,多年来人们一直在探索辅助策略,包括放疗、放化疗和化疗。从历史上看,很少有随机试验,其中包括各种其他肿瘤(例如胰腺和壶腹肿瘤);大多数证据依赖于 II 期研究和回顾性研究,没有高质量的证据来定义辅助策略的真正益处。 2017年以来,已报道三项随机III期临床试验;所有招募的胆道癌切除患者(CCA 和 GBC)被随机分配到单独观察组或吉西他滨化疗组(BCAT 研究;仅包括诊断为肝外 CCA 的患者)、吉西他滨和奥沙利铂组 (PRODIGE-12/ACCORD- 18;包括诊断为 CCA 和 GBC 的患者)或卡培他滨(BILCAP;包括诊断为 CCA 和 GBC 的患者)。 虽然基于吉西他滨的化疗未能显示出对患者预后(无复发生存期 (RFS) 或总生存期 (OS))的影响,但 BILCAP 研究显示辅助卡培他滨在 OS 方面具有益处(预先计划的敏感性分析)意向治疗人群和符合方案分析),在 RFS 方面已证实有益处。根据 BILCAP 试验,国际指南建议在可能治愈性切除 CCA 后使用卡培他滨辅助治疗 6 个月,作为目前切除 CCA 和 GBC 的护理标准。然而,BILCAP 未能在意向治疗(非敏感性分析)人群(主要终点)中显示 OS 获益,这一发现以及研究之间的一些不一致受到了批评,并导致了对胆道癌症医学界。本综述总结了胆道癌的辅助治疗领域,包括2017年前后的证据,以及最新随机III期研究之间的比较。对不同的发现提出了潜在的解释,并探讨了未来的步骤。
更新日期:2019-12-04
中文翻译:

胆道癌辅助治疗的当前标准和未来前景。
胆道癌,包括胆管癌(CCA)和胆囊癌(GBC)是罕见肿瘤,且发病率不断上升。预后很差,因为大多数患者被诊断为晚期疾病。只有约 20% 的患者被诊断为早期疾病,适合进行根治性手术。尽管手术具有潜在的治愈目的,但复发率很高,预计大约 60-70% 的患者会出现疾病复发。大多数复发以远处转移的形式发生,其中以肝脏扩散为主。鉴于肿瘤的高复发率,多年来人们一直在探索辅助策略,包括放疗、放化疗和化疗。从历史上看,很少有随机试验,其中包括各种其他肿瘤(例如胰腺和壶腹肿瘤);大多数证据依赖于 II 期研究和回顾性研究,没有高质量的证据来定义辅助策略的真正益处。 2017年以来,已报道三项随机III期临床试验;所有招募的胆道癌切除患者(CCA 和 GBC)被随机分配到单独观察组或吉西他滨化疗组(BCAT 研究;仅包括诊断为肝外 CCA 的患者)、吉西他滨和奥沙利铂组 (PRODIGE-12/ACCORD- 18;包括诊断为 CCA 和 GBC 的患者)或卡培他滨(BILCAP;包括诊断为 CCA 和 GBC 的患者)。 虽然基于吉西他滨的化疗未能显示出对患者预后(无复发生存期 (RFS) 或总生存期 (OS))的影响,但 BILCAP 研究显示辅助卡培他滨在 OS 方面具有益处(预先计划的敏感性分析)意向治疗人群和符合方案分析),在 RFS 方面已证实有益处。根据 BILCAP 试验,国际指南建议在可能治愈性切除 CCA 后使用卡培他滨辅助治疗 6 个月,作为目前切除 CCA 和 GBC 的护理标准。然而,BILCAP 未能在意向治疗(非敏感性分析)人群(主要终点)中显示 OS 获益,这一发现以及研究之间的一些不一致受到了批评,并导致了对胆道癌症医学界。本综述总结了胆道癌的辅助治疗领域,包括2017年前后的证据,以及最新随机III期研究之间的比较。对不同的发现提出了潜在的解释,并探讨了未来的步骤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号