当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Comprehensive Study on Self-Assembly and Gelation of C13-Dipeptides-From Design Strategies to Functionalities.
Biomacromolecules ( IF 5.5 ) Pub Date : 2019-12-16 , DOI: 10.1021/acs.biomac.9b01386 Tan Hu 1, 2 , Zhuo Zhang 1, 2 , Hao Hu 1, 2 , Stephen Robert Euston 3 , Siyi Pan 1, 2
Biomacromolecules ( IF 5.5 ) Pub Date : 2019-12-16 , DOI: 10.1021/acs.biomac.9b01386 Tan Hu 1, 2 , Zhuo Zhang 1, 2 , Hao Hu 1, 2 , Stephen Robert Euston 3 , Siyi Pan 1, 2
Affiliation
|
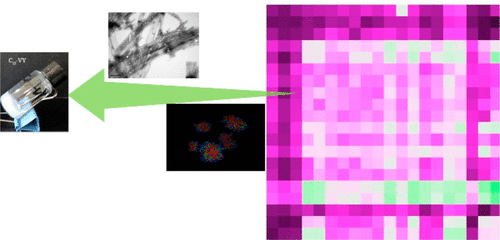
Computational and experimental methods were applied to investigate the self-assembly and gelation of C13-dipeptides. A modified aggregation propensity (APS) was introduced to correlate the effects of side chains of amino acids on the tendency to aggregate. From the experimental results, the ranges of 0.156 < APS < 0.250 seemed to be a proper region for the C13-dipeptides to form hydrogels, while other molecules with higher or lower APS were insoluble or dissociated. As observed from molecular dynamics simulations, the C13-dipeptides first formed small aggregates through hydrophobic interactions and then rearranged through electrostatic attractions and hydrogen bonds for self-assembly. The C13-dipeptides tended to be antiparallel packed, as shown by hydrogen bonding analyses. Experimental observations and analyses on the structures of C13-dipeptide hydrogels matched the computational conclusions very well. From the five selected gelators, i.e., C13-GW, C13-VY, and C13-WT, strong π-π stacking was observed. For C13-WS, strong hydrogen bonding was found, and in C13-WY, both strong π-π interactions and hydrogen bonds were found. It takes around 90 min or longer for C13-dipeptides to form hydrogels, and those formed by C13-WY and C13-WS had weak water holding capacities, which might be due to strong intermolecular hydrogen bonding. From rheological studies, the C13-dipeptides formed strong chemical gels that were stabilized by strong interactions between the molecular aggregates. These gelators exhibit the potentials to be environmentally friendly substitutes for the common functionalized peptide gelators.
中文翻译:

C13肽自组装和胶凝的全面研究-从设计策略到功能。
应用计算和实验方法研究了C13-二肽的自组装和凝胶化。引入了改进的聚集倾向(APS),以关联氨基酸侧链对聚集趋势的影响。从实验结果来看,0.156 <APS <0.250的范围似乎是C13-二肽形成水凝胶的合适区域,而其他具有较高或较低APS的分子则不溶或离解。从分子动力学模拟中观察到,C13-二肽首先通过疏水相互作用形成小的聚集体,然后通过静电引力和氢键进行重排以实现自组装。如氢键分析所示,C13-二肽倾向于反平行堆积。C13-二肽水凝胶结构的实验观察和分析与计算结论非常吻合。从五个选定的胶凝剂,即C13-GW,C13-VY和C13-WT中,观察到了强烈的π-π堆积。对于C13-WS,发现了很强的氢键,而在C13-WY中,发现了很强的π-π相互作用和氢键。C13-二肽形成水凝胶大约需要90分钟或更长时间,而由C13-WY和C13-WS形成的凝胶具有较弱的持水能力,这可能是由于强分子间氢键所致。根据流变学研究,C13-二肽形成了牢固的化学凝胶,这些化学凝胶通过分子聚集体之间的强相互作用而稳定了。这些胶凝剂具有潜在的环保功能,可替代常见的功能化肽胶凝剂。
更新日期:2019-12-17
中文翻译:

C13肽自组装和胶凝的全面研究-从设计策略到功能。
应用计算和实验方法研究了C13-二肽的自组装和凝胶化。引入了改进的聚集倾向(APS),以关联氨基酸侧链对聚集趋势的影响。从实验结果来看,0.156 <APS <0.250的范围似乎是C13-二肽形成水凝胶的合适区域,而其他具有较高或较低APS的分子则不溶或离解。从分子动力学模拟中观察到,C13-二肽首先通过疏水相互作用形成小的聚集体,然后通过静电引力和氢键进行重排以实现自组装。如氢键分析所示,C13-二肽倾向于反平行堆积。C13-二肽水凝胶结构的实验观察和分析与计算结论非常吻合。从五个选定的胶凝剂,即C13-GW,C13-VY和C13-WT中,观察到了强烈的π-π堆积。对于C13-WS,发现了很强的氢键,而在C13-WY中,发现了很强的π-π相互作用和氢键。C13-二肽形成水凝胶大约需要90分钟或更长时间,而由C13-WY和C13-WS形成的凝胶具有较弱的持水能力,这可能是由于强分子间氢键所致。根据流变学研究,C13-二肽形成了牢固的化学凝胶,这些化学凝胶通过分子聚集体之间的强相互作用而稳定了。这些胶凝剂具有潜在的环保功能,可替代常见的功能化肽胶凝剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号