当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Dynamic Switch in Inactive p38γ Leads to an Excited State on the Pathway to an Active Kinase.
Biochemistry ( IF 2.9 ) Pub Date : 2019-12-13 , DOI: 10.1021/acs.biochem.9b00932 Phillip C. Aoto , Robyn L. Stanfield , Ian A. Wilson , H. Jane Dyson , Peter E. Wright
Biochemistry ( IF 2.9 ) Pub Date : 2019-12-13 , DOI: 10.1021/acs.biochem.9b00932 Phillip C. Aoto , Robyn L. Stanfield , Ian A. Wilson , H. Jane Dyson , Peter E. Wright
|
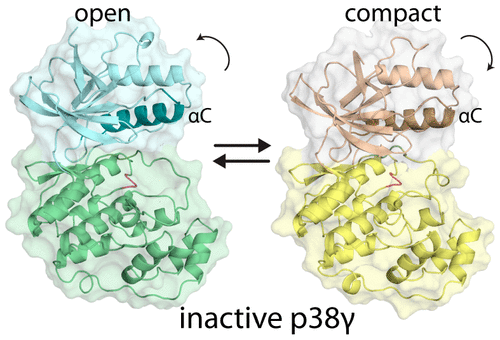
The inactive state of mitogen-activated protein kinases (MAPKs) adopts an open conformation while the active state exists in a compact form stabilized by phosphorylation. In the active state, eukaryotic kinases undergo breathing motions related to substrate binding and product release that have not previously been detected in the inactive state. However, docking interactions of partner proteins with inactive MAPK kinases exhibit allostery in binding of activating kinases. Interactions at a site distant from the activation loop are coupled to the configuration of the activation loop, suggesting that the inactive state may also undergo concerted dynamics. X-ray crystallographic studies of nonphosphorylated, inactive p38γ reveal differences in domain orientations and active site structure in the two molecules in the asymmetric unit. One molecule resembles an inactive kinase with an open active site. The second molecule has a rotation of the N-lobe that leads to partial compaction of the active site, resulting in a conformation that is intermediate between the inactive open state and the fully closed state of the activated kinase. Although the compact state of apo p38γ displays several of the features of the activated enzyme, it remains catalytically inert. In solution, the kinase fluctuates on a millisecond time scale between the open ground state and a weakly populated excited state that is similar in structure to the compact state observed in the crystal. The nuclear magnetic resonance and crystal structure data imply that interconversion between the open and compact states involves a molecular switch associated with the DFG loop.
中文翻译:

无效的p38γ中的动态开关会导致通往活性激酶途径的兴奋状态。
有丝分裂原活化蛋白激酶(MAPKs)的非活性状态采用开放构象,而活性状态以通过磷酸化作用稳定的紧密形式存在。在活性状态下,真核激酶经历与底物结合和产物释放有关的呼吸运动,而以前在非活性状态下尚未检测到这种运动。然而,伴侣蛋白与失活的MAPK激酶的对接相互作用在激活激酶的结合中表现出变构。远离激活环的站点上的交互作用与激活环的配置有关,这表明非活动状态也可能会发生协调的动态变化。对未磷酸化的非活性p38γ的X射线晶体学研究表明,不对称单元中两个分子的结构域方向和活性位点结构存在差异。一个分子类似于具有开放活性位点的失活激酶。第二个分子的N瓣旋转,导致活性位点部分压缩,从而导致构象介于激活的激酶的无活性开放状态和完全封闭状态之间。尽管载脂蛋白p38γ的致密状态显示了活化酶的几个特征,但仍保持催化惰性。在溶液中,激酶在开放基态和弱人口激发态之间以毫秒为单位的时间波动,该激发态的结构与晶体中观察到的致密态相似。核磁共振和晶体结构数据表明,开态和致密态之间的相互转换涉及与DFG环相关的分子开关。
更新日期:2019-12-17
中文翻译:

无效的p38γ中的动态开关会导致通往活性激酶途径的兴奋状态。
有丝分裂原活化蛋白激酶(MAPKs)的非活性状态采用开放构象,而活性状态以通过磷酸化作用稳定的紧密形式存在。在活性状态下,真核激酶经历与底物结合和产物释放有关的呼吸运动,而以前在非活性状态下尚未检测到这种运动。然而,伴侣蛋白与失活的MAPK激酶的对接相互作用在激活激酶的结合中表现出变构。远离激活环的站点上的交互作用与激活环的配置有关,这表明非活动状态也可能会发生协调的动态变化。对未磷酸化的非活性p38γ的X射线晶体学研究表明,不对称单元中两个分子的结构域方向和活性位点结构存在差异。一个分子类似于具有开放活性位点的失活激酶。第二个分子的N瓣旋转,导致活性位点部分压缩,从而导致构象介于激活的激酶的无活性开放状态和完全封闭状态之间。尽管载脂蛋白p38γ的致密状态显示了活化酶的几个特征,但仍保持催化惰性。在溶液中,激酶在开放基态和弱人口激发态之间以毫秒为单位的时间波动,该激发态的结构与晶体中观察到的致密态相似。核磁共振和晶体结构数据表明,开态和致密态之间的相互转换涉及与DFG环相关的分子开关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号