当前位置:
X-MOL 学术
›
Eur. J. Pharm. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fabrication of an osmotic 3D printed solid dosage form for controlled release of active pharmaceutical ingredients.
European Journal of Pharmaceutical Sciences ( IF 4.3 ) Pub Date : 2019-12-04 , DOI: 10.1016/j.ejps.2019.105176 Christos I Gioumouxouzis 1 , Emmanouil Tzimtzimis 2 , Orestis L Katsamenis 3 , Anthi Dourou 1 , Catherine Markopoulou 1 , Nikolaos Bouropoulos 4 , Dimitrios Tzetzis 2 , Dimitrios G Fatouros 1
European Journal of Pharmaceutical Sciences ( IF 4.3 ) Pub Date : 2019-12-04 , DOI: 10.1016/j.ejps.2019.105176 Christos I Gioumouxouzis 1 , Emmanouil Tzimtzimis 2 , Orestis L Katsamenis 3 , Anthi Dourou 1 , Catherine Markopoulou 1 , Nikolaos Bouropoulos 4 , Dimitrios Tzetzis 2 , Dimitrios G Fatouros 1
Affiliation
|
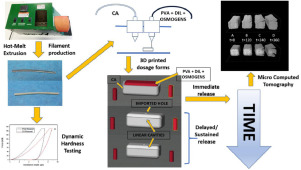
In pharmaceutical formulations, pharmacokinetic behavior of the Active Pharmaceutical Ingredients (API's) is significantly affected by their dissolution profiles. In this project, we attempted to create personalized dosage forms with osmotic properties that exhibit different API release patterns via Fused Deposition Modelling (FDM) 3D printing. Specifically, cellulose acetate was employed to create an external shell of an osmotically active core containing Diltiazem (DIL) as model drug. By removing parts of the shell (upper surface, linear lateral segments) were created dosage forms that modify their shape at specific time frames under the effect of the gradually induced osmotic pressure. Hot-Melt Extrusion (HME) was employed to fabricate two different 3DP feeding filaments, for the creation of either the shell or the osmotic core (dual-extrusion printing). Printed formulations and filaments were characterized by means of (TGA, XRD, DSC) and inspected using microscopy (optical and electron). The mechanical properties of the filaments were assessed by means of micro- and macro mechanical testing, whereas micro-Computed Tomography (μCT) was employed to investigate the volumetric changes occurring during the hydration process. XRD indicated the amorphization of DIL inside HME filaments and printed dosage forms, whereas the incorporated NaCl (osmogen) retained its crystallinity. Mechanical properties' testing confirmed the printability of produced filaments. Dissolution tests revealed that all formulations exhibited sustained release differing at the initiation time of the API dissolution (0, 120 and 360 min for the three different formulations). Finally, μCT uncovered the key structural changes associated with distinct phases of the release profile. The above results demonstrate the successful utilization of an FDM 3D printer in order to create osmotic 3D printed formulations exhibiting sustained and/or delayed release, that can be easily personalized containing API doses corresponding to each patient's specific needs.
中文翻译:

渗透性3D打印固体剂型的制备,用于控制活性药物成分的释放。
在药物制剂中,活性药物成分(API)的药代动力学行为会受到其溶出度的显着影响。在这个项目中,我们尝试通过熔融沉积建模(FDM)3D打印创建具有渗透性的个性化剂型,这些剂型具有不同的API释放模式。具体地,使用乙酸纤维素来产生包含地尔硫卓(DIL)作为模型药物的渗透活性核的外壳。通过除去壳的部分(上表面,线性侧链段),形成剂型,该剂型在逐渐诱导的渗透压的作用下,在特定的时间范围内改变其形状。采用热熔挤出(HME)来制造两条不同的3DP进料丝,用于创建外壳或渗透核(双挤压印刷)。通过(TGA,XRD,DSC)表征印刷的制剂和长丝,并使用显微镜(光学和电子)检查。细丝的机械性能通过微观和宏观力学测试进行了评估,而显微计算机断层扫描(μCT)用于研究水合过程中发生的体积变化。X射线衍射表明HME细丝和印刷剂型中DIL的非晶化,而掺入的NaCl(渗透压)保持其结晶度。机械性能的测试证实了生产的长丝的可印刷性。溶出度测试表明,所有制剂在API溶出开始时均表现出不同的持续释放(0,三种不同的配方分别为120分钟和360分钟)。最后,μCT发现了与释放曲线不同阶段相关的关键结构变化。以上结果证明了FDM 3D打印机的成功利用,以创建具有持续释放和/或延迟释放的渗透性3D打印制剂,可以轻松地个性化包含与每个患者的特定需求相对应的API剂量。
更新日期:2019-12-04
中文翻译:

渗透性3D打印固体剂型的制备,用于控制活性药物成分的释放。
在药物制剂中,活性药物成分(API)的药代动力学行为会受到其溶出度的显着影响。在这个项目中,我们尝试通过熔融沉积建模(FDM)3D打印创建具有渗透性的个性化剂型,这些剂型具有不同的API释放模式。具体地,使用乙酸纤维素来产生包含地尔硫卓(DIL)作为模型药物的渗透活性核的外壳。通过除去壳的部分(上表面,线性侧链段),形成剂型,该剂型在逐渐诱导的渗透压的作用下,在特定的时间范围内改变其形状。采用热熔挤出(HME)来制造两条不同的3DP进料丝,用于创建外壳或渗透核(双挤压印刷)。通过(TGA,XRD,DSC)表征印刷的制剂和长丝,并使用显微镜(光学和电子)检查。细丝的机械性能通过微观和宏观力学测试进行了评估,而显微计算机断层扫描(μCT)用于研究水合过程中发生的体积变化。X射线衍射表明HME细丝和印刷剂型中DIL的非晶化,而掺入的NaCl(渗透压)保持其结晶度。机械性能的测试证实了生产的长丝的可印刷性。溶出度测试表明,所有制剂在API溶出开始时均表现出不同的持续释放(0,三种不同的配方分别为120分钟和360分钟)。最后,μCT发现了与释放曲线不同阶段相关的关键结构变化。以上结果证明了FDM 3D打印机的成功利用,以创建具有持续释放和/或延迟释放的渗透性3D打印制剂,可以轻松地个性化包含与每个患者的特定需求相对应的API剂量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号