Molecular Catalysis ( IF 3.9 ) Pub Date : 2019-12-04 , DOI: 10.1016/j.mcat.2019.110694 Marcela M.L. Tormena , Rodrigo M. Pontes
|
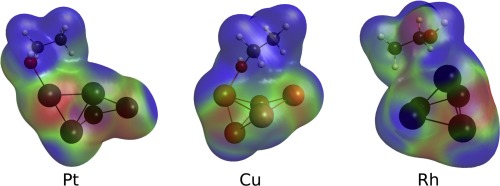
Ethanol reforming has emerged in the last decades as a powerful solution for the energy problem, providing a way for hydrogen production from a renewable and environmentally friendly source. In the search for the best catalyst for this process, it is crucial to understand the reaction mechanism in all its details. In this work, we compare the reaction mechanism for the ethanol decomposition over Pt, Cu and Rh. The approach presented here involves the use of metal clusters treated by effective core potential basis set and the remaining species treated with all electrons. DFT calculations (B3LYP-D3) were used to construct the potential energy surface for all the steps in the path from ethanol to CH4, CH3CH3 and CH2CH2. Canonical molecular orbital energy decomposition analysis (CMOEDA) was used to understand the distinct behavior of the three metals. After the formation of acetaldehyde, the reaction over Pt and Rh easily continues towards the C–C bond breaking, which is much more difficult for Cu. The better capability of Pt and Rh to stabilize transition states is, in most cases, explained by a more malleable electron density in these clusters. This malleability allows the clusters to adjust the electron density to better deal with charge transfers taking place throughout every reaction step.
中文翻译:

DFT / EDA研究Pt,Cu和Rh金属簇上乙醇的分解
乙醇重整作为能源问题的有力解决方案,在过去的几十年中出现了,为从可再生和环境友好的来源生产氢气提供了一种途径。在寻找该方法的最佳催化剂时,至关重要的是要全面了解反应机理。在这项工作中,我们比较了乙醇在Pt,Cu和Rh上分解的反应机理。此处介绍的方法涉及使用通过有效核心电势基集处理的金属簇,以及使用所有电子处理的其余物种。使用DFT计算(B3LYP-D3)构造从乙醇到CH 4,CH 3 CH 3和CH 2 CH 2路径中所有步骤的势能面。使用规范分子轨道能量分解分析(CMOEDA)来了解这三种金属的不同行为。乙醛形成后,Pt和Rh上的反应很容易继续朝C–C键断裂,这对Cu来说要困难得多。在大多数情况下,Pt和Rh具有更好的稳定过渡态的能力,可以通过这些簇中具有更大延展性的电子密度来解释。这种可延展性允许簇调节电子密度以更好地处理在每个反应步骤中发生的电荷转移。











































 京公网安备 11010802027423号
京公网安备 11010802027423号