Molecular Catalysis ( IF 3.9 ) Pub Date : 2019-12-04 , DOI: 10.1016/j.mcat.2019.110723 Qiuli Liu , Yang Wang , Yanyan Wang , Xue Li , Ling-Bo Qu , Yu Lan , Donghui Wei
|
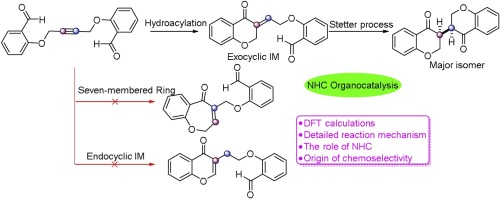
The regioselectivity of hydroacylation mediated by transition metal and organocatalyst has been and continues to be one of the most challenging questions in the synthesis field. To explore the possible mechanisms and origin of the regio-/enantioselectivity, the density functional theory (DFT) calculations were performed for studying intramolecular hydroacylation-Stetter reaction of alkynyl bisbenzaldehyde catalyzed by N-heterocyclic carbene (NHC). Computational results show that the hydroacylation process was the rate-determining step and irreversible. Four different stereoisomers (RR, RS, SR, and SS) were considered and the lowest energy mode agreed with the reported enantioselectivity in experiment. Global reaction index (GRI) analysis showed that NHC catalyst can convert alkynyl bisbenzaldehyde electrophilic carbonyl carbon into nucleophilicity to initiate the reaction. Moreover, electron localization function (ELF) and intrinsic reaction coordinate (IRC) analyses were performed to characterize the manner difference between the process of hydroacylation and Stetter reaction.
中文翻译:

N-杂环卡宾(NHC)催化的分子内加氢-键结反应级联反应的计算研究
由过渡金属和有机催化剂介导的加氢酰化的区域选择性一直并且继续是合成领域中最具挑战性的问题之一。为了探索区域-对映体选择性的可能机理和起源,进行了密度泛函理论(DFT)计算,以研究N-杂环卡宾(NHC)催化的炔基双苯甲醛的分子内加氢酰化-Stetter反应。计算结果表明加氢酰化过程是决定速率的步骤,并且是不可逆的。考虑了四种不同的立体异构体(RR,RS,SR和SS),最低能量模式与实验中报道的对映选择性一致。全局反应指数(GRI)分析表明,NHC催化剂可将炔基双苯甲醛亲电子羰基碳转化为亲核性,从而引发反应。此外,进行了电子本地化功能(ELF)和本征反应坐标(IRC)分析,以表征加氢酰化和Stetter反应之间的方式差异。











































 京公网安备 11010802027423号
京公网安备 11010802027423号