The Lancet Respiratory Medicine ( IF 38.7 ) Pub Date : 2019-12-03 , DOI: 10.1016/s2213-2600(19)30367-4 Thomas P Hellyer 1 , Daniel F McAuley 2 , Timothy S Walsh 3 , Niall Anderson 4 , Andrew Conway Morris 5 , Suveer Singh 6 , Paul Dark 7 , Alistair I Roy 8 , Gavin D Perkins 9 , Ronan McMullan 10 , Lydia M Emerson 10 , Bronagh Blackwood 10 , Stephen E Wright 11 , Kallirroi Kefala 12 , Cecilia M O'Kane 10 , Simon V Baudouin 13 , Ross L Paterson 14 , Anthony J Rostron 15 , Ashley Agus 16 , Jonathan Bannard-Smith 17 , Nicole M Robin 18 , Ingeborg D Welters 19 , Christopher Bassford 20 , Bryan Yates 21 , Craig Spencer 22 , Shondipon K Laha 22 , Jonathan Hulme 23 , Stephen Bonner 24 , Vanessa Linnett 25 , Julian Sonksen 26 , Tina Van Den Broeck 27 , Gert Boschman 27 , Dw James Keenan 27 , Jonathan Scott 1 , A Joy Allen 28 , Glenn Phair 16 , Jennie Parker 29 , Susan A Bowett 29 , A John Simpson 30
|
Background
Ventilator-associated pneumonia is the most common intensive care unit (ICU)-acquired infection, yet accurate diagnosis remains difficult, leading to overuse of antibiotics. Low concentrations of IL-1β and IL-8 in bronchoalveolar lavage fluid have been validated as effective markers for exclusion of ventilator-associated pneumonia. The VAPrapid2 trial aimed to determine whether measurement of bronchoalveolar lavage fluid IL-1β and IL-8 could effectively and safely improve antibiotic stewardship in patients with clinically suspected ventilator-associated pneumonia.
Methods
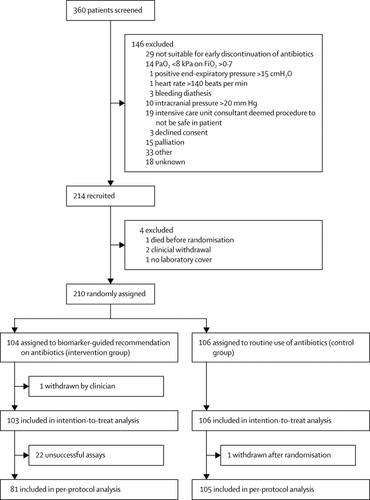
VAPrapid2 was a multicentre, randomised controlled trial in patients admitted to 24 ICUs from 17 National Health Service hospital trusts across England, Scotland, and Northern Ireland. Patients were screened for eligibility and included if they were 18 years or older, intubated and mechanically ventilated for at least 48 h, and had suspected ventilator-associated pneumonia. Patients were randomly assigned (1:1) to biomarker-guided recommendation on antibiotics (intervention group) or routine use of antibiotics (control group) using a web-based randomisation service hosted by Newcastle Clinical Trials Unit. Patients were randomised using randomly permuted blocks of size four and six and stratified by site, with allocation concealment. Clinicians were masked to patient assignment for an initial period until biomarker results were reported. Bronchoalveolar lavage was done in all patients, with concentrations of IL-1β and IL-8 rapidly determined in bronchoalveolar lavage fluid from patients randomised to the biomarker-based antibiotic recommendation group. If concentrations were below a previously validated cutoff, clinicians were advised that ventilator-associated pneumonia was unlikely and to consider discontinuing antibiotics. Patients in the routine use of antibiotics group received antibiotics according to usual practice at sites. Microbiology was done on bronchoalveolar lavage fluid from all patients and ventilator-associated pneumonia was confirmed by at least 104 colony forming units per mL of bronchoalveolar lavage fluid. The primary outcome was the distribution of antibiotic-free days in the 7 days following bronchoalveolar lavage. Data were analysed on an intention-to-treat basis, with an additional per-protocol analysis that excluded patients randomly assigned to the intervention group who defaulted to routine use of antibiotics because of failure to return an adequate biomarker result. An embedded process evaluation assessed factors influencing trial adoption, recruitment, and decision making. This study is registered with ISRCTN, ISRCTN65937227, and ClinicalTrials.gov, NCT01972425.
Findings
Between Nov 6, 2013, and Sept 13, 2016, 360 patients were screened for inclusion in the study. 146 patients were ineligible, leaving 214 who were recruited to the study. Four patients were excluded before randomisation, meaning that 210 patients were randomly assigned to biomarker-guided recommendation on antibiotics (n=104) or routine use of antibiotics (n=106). One patient in the biomarker-guided recommendation group was withdrawn by the clinical team before bronchoscopy and so was excluded from the intention-to-treat analysis. We found no significant difference in the primary outcome of the distribution of antibiotic-free days in the 7 days following bronchoalveolar lavage in the intention-to-treat analysis (p=0·58). Bronchoalveolar lavage was associated with a small and transient increase in oxygen requirements. Established prescribing practices, reluctance for bronchoalveolar lavage, and dependence on a chain of trial-related procedures emerged as factors that impaired trial processes.
Interpretation
Antibiotic use remains high in patients with suspected ventilator-associated pneumonia. Antibiotic stewardship was not improved by a rapid, highly sensitive rule-out test. Prescribing culture, rather than poor test performance, might explain this absence of effect.
Funding
UK Department of Health and the Wellcome Trust.
中文翻译:

生物标志物引导的疑似呼吸机相关性肺炎抗生素管理(VAPrapid2):一项随机对照试验和过程评估。
背景
呼吸机相关性肺炎是最常见的重症监护病房 (ICU) 获得性感染,但准确诊断仍然很困难,导致抗生素的过度使用。支气管肺泡灌洗液中低浓度的 IL-1β 和 IL-8 已被证实是排除呼吸机相关性肺炎的有效标志物。 VAPrapid2 试验旨在确定支气管肺泡灌洗液 IL-1β 和 IL-8 的测量是否可以有效、安全地改善临床疑似呼吸机相关性肺炎患者的抗生素管理。
方法
VAPrapid2 是一项多中心、随机对照试验,受试者为来自英格兰、苏格兰和北爱尔兰 17 个国家卫生服务信托医院 24 个 ICU 的患者。对患者进行资格筛选,如果患者年满 18 岁、插管并机械通气至少 48 小时,并且疑似患有呼吸机相关性肺炎,则纳入研究。使用纽卡斯尔临床试验单位主办的基于网络的随机化服务,将患者随机分配 (1:1) 至生物标志物指导的抗生素推荐组(干预组)或常规使用抗生素组(对照组)。使用大小为 4 和 6 的随机排列块对患者进行随机分组,并按部位进行分层,并进行分配隐藏。在报告生物标志物结果之前,临床医生最初对患者分配情况不知情。所有患者均进行支气管肺泡灌洗,并快速测定随机分配至基于生物标志物的抗生素推荐组的患者的支气管肺泡灌洗液中 IL-1β 和 IL-8 的浓度。如果浓度低于先前验证的临界值,则建议临床医生不太可能出现呼吸机相关性肺炎,并考虑停用抗生素。常规使用抗生素组患者按照现场常规使用抗生素。对所有患者的支气管肺泡灌洗液进行微生物学检查,每毫升支气管肺泡灌洗液至少有 10 4 个菌落形成单位,证实呼吸机相关性肺炎。主要结果是支气管肺泡灌洗后 7 天内无抗生素天数的分布。 数据以意向治疗为基础进行分析,并进行了额外的按方案分析,排除了随机分配到干预组的患者,这些患者由于未能返回足够的生物标志物结果而默认常规使用抗生素。嵌入式流程评估评估了影响试验采用、招募和决策的因素。本研究已在 ISRCTN(ISRCTN65937227)和 ClinicalTrials.gov(NCT01972425)注册。
发现
2013年11月6日至2016年9月13日期间,筛选了360名患者纳入该研究。 146 名患者不符合资格,剩下 214 名患者被招募参加该研究。随机分组前排除了 4 名患者,这意味着 210 名患者被随机分配到生物标志物指导的抗生素推荐组 (n=104) 或常规使用抗生素组 (n=106)。生物标志物指导推荐组中的一名患者在支气管镜检查前被临床团队撤回,因此被排除在意向治疗分析之外。在意向治疗分析中,我们发现支气管肺泡灌洗后 7 天内无抗生素天数分布的主要结果没有显着差异 (p=0·58)。支气管肺泡灌洗与氧气需求的小幅短暂增加有关。既定的处方实践、不愿进行支气管肺泡灌洗以及对一系列试验相关程序的依赖成为损害试验过程的因素。
解释
疑似呼吸机相关性肺炎患者的抗生素使用率仍然很高。快速、高度敏感的排除测试并没有改善抗生素管理。处方培养,而不是糟糕的测试表现,可能可以解释这种效果缺失的原因。
资金
英国卫生部和威康信托基金会。









































 京公网安备 11010802027423号
京公网安备 11010802027423号