当前位置:
X-MOL 学术
›
Lancet Oncol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): a phase 3, multicentre, randomised, controlled, open-label study.
The Lancet Oncology ( IF 51.1 ) Pub Date : 2019-12-03 , DOI: 10.1016/s1470-2045(19)30735-1 Brian I Rini 1 , Sumanta K Pal 2 , Bernard J Escudier 3 , Michael B Atkins 4 , Thomas E Hutson 5 , Camillo Porta 6 , Elena Verzoni 7 , Michael N Needle 8 , David F McDermott 9
The Lancet Oncology ( IF 51.1 ) Pub Date : 2019-12-03 , DOI: 10.1016/s1470-2045(19)30735-1 Brian I Rini 1 , Sumanta K Pal 2 , Bernard J Escudier 3 , Michael B Atkins 4 , Thomas E Hutson 5 , Camillo Porta 6 , Elena Verzoni 7 , Michael N Needle 8 , David F McDermott 9
Affiliation
|
BACKGROUND
Treatment for renal cell carcinoma has been revolutionised by inhibitors of VEGF receptor. Previous studies have suggested that treatment with a VEGF receptor (VEGFR) tyrosine kinase inhibitor might be effective in patients who had previous checkpoint inhibitor therapy. Therefore, TIVO-3 was designed to compare the efficacy and safety of tivozanib (a potent and selective VEGFR inhibitor) with those of sorafenib as third-line or fourth-line therapy in patients with metastatic renal cell carcinoma.
METHODS
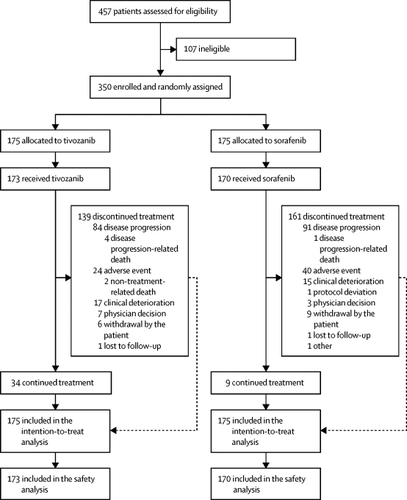
In this open-label, randomised, controlled trial done at 120 academic hospitals in 12 countries, we enrolled eligible patients older than 18 years with histologically or cytologically confirmed metastatic renal cell carcinoma and at least two previous systemic treatments (including at least one previous treatment with a VEGFR inhibitor), measurable disease according to the Response Evaluation Criteria in Solid Tumors version 1.1, and an Eastern Cooperative Oncology Group performance status of 0 or 1. Patients were excluded if they had received previous treatment with tivozanib or sorafenib. Patients were stratified by International Metastatic Renal Cell Carcinoma Database Consortium risk category and type of previous therapy and randomised (1:1) with a complete permuted block design (block size of four) to either tivozanib 1·5 mg orally once daily in 4-week cycles or sorafenib 400 mg orally twice daily continuously. Investigators and patients were not masked to treatment. The primary endpoint was progression-free survival by independent review in the intention-to-treat population. Safety analyses were done in all patients who received at least one dose of study treatment. This trial is registered with ClinicalTrials.gov, NCT02627963.
FINDINGS
Between May 24, 2016, and Aug 14, 2017, 350 patients were randomly assigned to receive tivozanib (175 patients) or sorafenib (175 patients). Median follow-up was 19·0 months (IQR 15·0-23·4). Median progression-free survival was significantly longer with tivozanib (5·6 months, 95% CI 5·29-7·33) than with sorafenib (3·9 months, 3·71-5·55; hazard ratio 0·73, 95% CI 0·56-0·94; p=0·016). The most common grade 3 or 4 treatment-related adverse event was hypertension (35 [20%] of 173 patients treated with tivozanib and 23 [14%] of 170 patients treated with sorafenib). Serious treatment-related adverse events occurred in 19 (11%) patients with tivozanib and in 17 (10%) patients with sorafenib. No treatment-related deaths were reported.
INTERPRETATION
Our study showed that tivozanib as third-line or fourth-line therapy improved progression-free survival and was better tolerated compared with sorafenib in patients with metastatic renal cell carcinoma.
FUNDING
AVEO Oncology.
中文翻译:

晚期肾细胞癌(TIVO-3)患者的替沃扎尼vs索拉非尼:3期,多中心,随机,对照,开放标签研究。
背景技术肾细胞癌的治疗已被VEGF受体抑制剂彻底革新。先前的研究表明,使用VEGF受体(VEGFR)酪氨酸激酶抑制剂治疗可能对先前接受过检查点抑制剂治疗的患者有效。因此,TIVO-3设计用于比较替沃扎尼(一种有效的选择性VEGFR抑制剂)与索拉非尼作为三线或四线治疗转移性肾细胞癌的疗效和安全性。方法在这项开放性,随机,对照试验中,该试验在12个国家/地区的120家学术医院中进行,根据1.1版《实体瘤反应评估标准》,我们招募了18岁以上经组织学或细胞学证实为转移性肾细胞癌且至少接受过两次全身性治疗(包括至少接受过VEGFR抑制剂治疗)且可测量的疾病的合格患者。 ,并且东部合作肿瘤小组的工作状态为0或1。如果患者先前曾接受过tivozanib或sorafenib的治疗,则将其排除在外。根据国际转移性肾细胞癌数据库联合会的风险类别和既往治疗类型对患者进行分层,并采用完全置换的区组设计(区组大小为四个)随机分配(1:1)至替沃扎尼1·5 mg,每天口服一次,共4次。一周周期或索拉非尼400 mg口服,每天连续两次。研究者和患者没有被掩盖治疗。主要终点是通过独立评估意向性治疗人群的无进展生存期。对接受至少一剂研究治疗药物的所有患者进行安全性分析。该试验已在ClinicalTrials.gov上注册,NCT02627963。结果在2016年5月24日至2017年8月14日之间,将350名患者随机分配接受替沃扎尼(175例)或索拉非尼(175例)。中位随访时间为19·0个月(IQR 15·0-23·4)。替沃扎尼(5·6个月,95%CI 5·29-7·33)的中位无进展生存期显着长于索拉非尼(3·9个月,3·71-5·55;危险比0·73, 95%CI 0·56-0·94; p = 0·016)。与治疗相关的最常见的3级或4级不良事件是高血压(接受替沃扎尼治疗的173例患者中35例[20%],接受索拉非尼治疗的170例患者23例[14%])。严重的与治疗相关的不良事件发生在tivozanib的19例患者(11%)和sorafenib的17例(10%)患者中。没有报道与治疗有关的死亡。解释我们的研究表明,与索拉非尼相比,替沃扎尼作为三线或四线治疗可改善无进展生存期,并且对转移性肾细胞癌患者的耐受性更好。资助AVEO肿瘤学。解释我们的研究表明,与索拉非尼相比,替沃扎尼作为三线或四线疗法可改善无进展生存期,并且对转移性肾细胞癌患者的耐受性更好。资助AVEO肿瘤学。解释我们的研究表明,与索拉非尼相比,替沃扎尼作为三线或四线疗法可改善无进展生存期,并且对转移性肾细胞癌患者的耐受性更好。资助AVEO肿瘤学。
更新日期:2020-01-04
中文翻译:

晚期肾细胞癌(TIVO-3)患者的替沃扎尼vs索拉非尼:3期,多中心,随机,对照,开放标签研究。
背景技术肾细胞癌的治疗已被VEGF受体抑制剂彻底革新。先前的研究表明,使用VEGF受体(VEGFR)酪氨酸激酶抑制剂治疗可能对先前接受过检查点抑制剂治疗的患者有效。因此,TIVO-3设计用于比较替沃扎尼(一种有效的选择性VEGFR抑制剂)与索拉非尼作为三线或四线治疗转移性肾细胞癌的疗效和安全性。方法在这项开放性,随机,对照试验中,该试验在12个国家/地区的120家学术医院中进行,根据1.1版《实体瘤反应评估标准》,我们招募了18岁以上经组织学或细胞学证实为转移性肾细胞癌且至少接受过两次全身性治疗(包括至少接受过VEGFR抑制剂治疗)且可测量的疾病的合格患者。 ,并且东部合作肿瘤小组的工作状态为0或1。如果患者先前曾接受过tivozanib或sorafenib的治疗,则将其排除在外。根据国际转移性肾细胞癌数据库联合会的风险类别和既往治疗类型对患者进行分层,并采用完全置换的区组设计(区组大小为四个)随机分配(1:1)至替沃扎尼1·5 mg,每天口服一次,共4次。一周周期或索拉非尼400 mg口服,每天连续两次。研究者和患者没有被掩盖治疗。主要终点是通过独立评估意向性治疗人群的无进展生存期。对接受至少一剂研究治疗药物的所有患者进行安全性分析。该试验已在ClinicalTrials.gov上注册,NCT02627963。结果在2016年5月24日至2017年8月14日之间,将350名患者随机分配接受替沃扎尼(175例)或索拉非尼(175例)。中位随访时间为19·0个月(IQR 15·0-23·4)。替沃扎尼(5·6个月,95%CI 5·29-7·33)的中位无进展生存期显着长于索拉非尼(3·9个月,3·71-5·55;危险比0·73, 95%CI 0·56-0·94; p = 0·016)。与治疗相关的最常见的3级或4级不良事件是高血压(接受替沃扎尼治疗的173例患者中35例[20%],接受索拉非尼治疗的170例患者23例[14%])。严重的与治疗相关的不良事件发生在tivozanib的19例患者(11%)和sorafenib的17例(10%)患者中。没有报道与治疗有关的死亡。解释我们的研究表明,与索拉非尼相比,替沃扎尼作为三线或四线治疗可改善无进展生存期,并且对转移性肾细胞癌患者的耐受性更好。资助AVEO肿瘤学。解释我们的研究表明,与索拉非尼相比,替沃扎尼作为三线或四线疗法可改善无进展生存期,并且对转移性肾细胞癌患者的耐受性更好。资助AVEO肿瘤学。解释我们的研究表明,与索拉非尼相比,替沃扎尼作为三线或四线疗法可改善无进展生存期,并且对转移性肾细胞癌患者的耐受性更好。资助AVEO肿瘤学。



























 京公网安备 11010802027423号
京公网安备 11010802027423号