当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermokinetic Analysis of Protein Subunit Exchange by Variable-Temperature Native Mass Spectrometry.
Biochemistry ( IF 2.9 ) Pub Date : 2019-12-04 , DOI: 10.1021/acs.biochem.9b00911 Senthil K Thangaraj 1 , Salman James 1 , Juha Rouvinen 1 , Janne Jänis 1
Biochemistry ( IF 2.9 ) Pub Date : 2019-12-04 , DOI: 10.1021/acs.biochem.9b00911 Senthil K Thangaraj 1 , Salman James 1 , Juha Rouvinen 1 , Janne Jänis 1
Affiliation
|
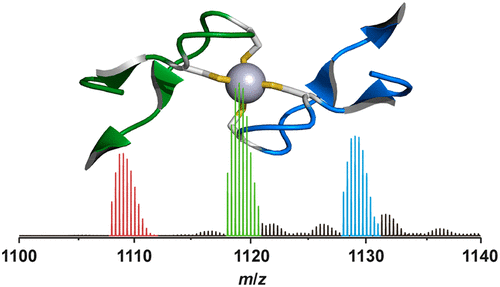
Many protein complexes are assembled from a varying number of subunits, which are continuously exchanging with diverse time scales. This structural dynamics is considered to be important for many regulatory and sensory adaptation processes that occur in vivo. We have developed an accurate method for monitoring protein subunit exchange by using native electrospray ionization mass spectrometry (ESI-MS), exemplified here for an extremely stable Rad50 zinc hook (Hk) dimer assembly, Zn(Hk)2. The method has two steps: appropriate protein/peptide mutation and native ESI-MS analysis using a variable-temperature sample inlet. In this work, two Hk mutants were produced, mixed with wild-type Hk, and measured at three different temperatures. A thermokinetic analysis of heterodimer formation allowed us to determine the enthalpy, entropy, and Gibbs free energy of activation for subunit exchange, showing that the reaction is slow and associated with a high enthalpic barrier, consistent with the exceptionally high stability of the Zn(Hk)2 assembly.
中文翻译:

可变温度天然质谱分析蛋白质亚基交换的热动力学分析。
许多蛋白质复合物是由不同数量的亚基组装而成的,这些亚基在不同的时间尺度上不断交换。对于体内发生的许多调节和感觉适应过程,这种结构动力学被认为是重要的。我们已经开发了一种通过使用天然电喷雾电离质谱(ESI-MS)监测蛋白质亚基交换的准确方法,此处以极稳定的Rad50锌钩(Hk)二聚体组件Zn(Hk)2为例。该方法包括两个步骤:适当的蛋白质/肽突变和使用可变温度样品入口的天然ESI-MS分析。在这项工作中,产生了两个Hk突变体,与野生型Hk混合,并在三个不同的温度下进行了测量。对异二聚体形成的热动力学分析使我们能够确定焓,熵,
更新日期:2019-12-04
中文翻译:

可变温度天然质谱分析蛋白质亚基交换的热动力学分析。
许多蛋白质复合物是由不同数量的亚基组装而成的,这些亚基在不同的时间尺度上不断交换。对于体内发生的许多调节和感觉适应过程,这种结构动力学被认为是重要的。我们已经开发了一种通过使用天然电喷雾电离质谱(ESI-MS)监测蛋白质亚基交换的准确方法,此处以极稳定的Rad50锌钩(Hk)二聚体组件Zn(Hk)2为例。该方法包括两个步骤:适当的蛋白质/肽突变和使用可变温度样品入口的天然ESI-MS分析。在这项工作中,产生了两个Hk突变体,与野生型Hk混合,并在三个不同的温度下进行了测量。对异二聚体形成的热动力学分析使我们能够确定焓,熵,











































 京公网安备 11010802027423号
京公网安备 11010802027423号