当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigations of Albumin-Insulin Detemir Complexes Using Molecular Dynamics Simulations and Free Energy Calculations.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2019-12-16 , DOI: 10.1021/acs.molpharmaceut.9b00839 Line A Ryberg 1 , Pernille Sønderby 1 , Jens T Bukrinski 2 , Pernille Harris 1 , Günther H J Peters 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2019-12-16 , DOI: 10.1021/acs.molpharmaceut.9b00839 Line A Ryberg 1 , Pernille Sønderby 1 , Jens T Bukrinski 2 , Pernille Harris 1 , Günther H J Peters 1
Affiliation
|
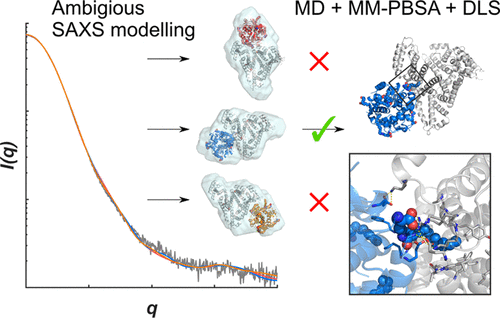
Insulin detemir is a lipidated insulin analogue that obtains a half-life extension by oligomerization and reversible binding to human serum albumin. In the present study, the complex between a detemir hexamer and albumin is investigated by an integrative approach combining molecular dynamics (MD) simulations, molecular mechanics Poisson-Boltzmann surface area (MM-PBSA) free energy calculations, and dynamic light scattering (DLS) experiments. Recent reported small-angle X-ray scattering data could not unambiguously resolve the exact binding site of detemir on albumin. We therefore applied MD simulations to deduce the binding site and key protein-protein interactions. MD simulations were started from initial complex structures based on the SAXS models, and free energies of binding were estimated from the simulations by using the MM-PBSA approach for the different binding positions. The results suggest that the overlapping FA3-FA4 binding site (named FA4) is the most favorable site with a calculated free energy of binding of -28 ± 6 kcal/mol and a good fit to the reported SAXS data throughout the simulations. Multiple salt bridges, hydrogen bonds, and favorable van der Waals interactions are observed in the binding interface that promote complexation. The binding to FA4 is further supported by DLS competition experiments with the prototypical FA4 ligand, ibuprofen, showing displacement of detemir by ibuprofen. This study provides information on albumin-detemir binding on a molecular level, which could be utilized in a rational design of future lipidated albumin-binding peptides.
中文翻译:

使用分子动力学模拟和自由能计算研究白蛋白-胰岛素德特米尔复合物。
地特胰岛素是一种脂质化的胰岛素类似物,可通过低聚和与人血清白蛋白的可逆结合而延长半衰期。在本研究中,通过结合分子动力学(MD)模拟,分子力学泊松-玻尔兹曼表面积(MM-PBSA)自由能计算和动态光散射(DLS)的综合方法研究了Detemir六聚体与白蛋白之间的复合物。实验。最近报道的小角度X射线散射数据无法明确解析德特米尔在白蛋白上的确切结合位点。因此,我们应用MD模拟来推断结合位点和关键的蛋白质-蛋白质相互作用。MD模拟是从基于SAXS模型的初始复杂结构开始的,通过使用MM-PBSA方法对不同的结合位置进行模拟,可以估算结合的自由能和结合能。结果表明,重叠的FA3-FA4结合位点(命名为FA4)是最有利的位点,其计算的结合自由能为-28±6 kcal / mol,与整个模拟过程中报告的SAXS数据非常吻合。在促进络合的结合界面中观察到多个盐桥,氢键和有利的范德华相互作用。与FA4的结合由具有原型FA4配体布洛芬的DLS竞争实验进一步支持,显示布洛芬取代了德特米尔。这项研究在分子水平上提供了有关白蛋白-德特米尔结合的信息,可用于未来脂化白蛋白结合肽的合理设计中。
更新日期:2019-12-17
中文翻译:

使用分子动力学模拟和自由能计算研究白蛋白-胰岛素德特米尔复合物。
地特胰岛素是一种脂质化的胰岛素类似物,可通过低聚和与人血清白蛋白的可逆结合而延长半衰期。在本研究中,通过结合分子动力学(MD)模拟,分子力学泊松-玻尔兹曼表面积(MM-PBSA)自由能计算和动态光散射(DLS)的综合方法研究了Detemir六聚体与白蛋白之间的复合物。实验。最近报道的小角度X射线散射数据无法明确解析德特米尔在白蛋白上的确切结合位点。因此,我们应用MD模拟来推断结合位点和关键的蛋白质-蛋白质相互作用。MD模拟是从基于SAXS模型的初始复杂结构开始的,通过使用MM-PBSA方法对不同的结合位置进行模拟,可以估算结合的自由能和结合能。结果表明,重叠的FA3-FA4结合位点(命名为FA4)是最有利的位点,其计算的结合自由能为-28±6 kcal / mol,与整个模拟过程中报告的SAXS数据非常吻合。在促进络合的结合界面中观察到多个盐桥,氢键和有利的范德华相互作用。与FA4的结合由具有原型FA4配体布洛芬的DLS竞争实验进一步支持,显示布洛芬取代了德特米尔。这项研究在分子水平上提供了有关白蛋白-德特米尔结合的信息,可用于未来脂化白蛋白结合肽的合理设计中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号