当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Measurement of Hepatic ABCB1 and ABCG2 Transport Activity with [11C]Tariquidar and PET in Humans and Mice.
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2019-12-17 , DOI: 10.1021/acs.molpharmaceut.9b01060 Irene Hernández Lozano 1 , Martin Bauer 1 , Beatrix Wulkersdorfer 1 , Alexander Traxl 2 , Cécile Philippe 3 , Maria Weber 1 , Stephanie Häusler 4 , Bruno Stieger 4 , Walter Jäger 5 , Severin Mairinger 2 , Thomas Wanek 2 , Marcus Hacker 3 , Markus Zeitlinger 1 , Oliver Langer 1, 2, 3
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2019-12-17 , DOI: 10.1021/acs.molpharmaceut.9b01060 Irene Hernández Lozano 1 , Martin Bauer 1 , Beatrix Wulkersdorfer 1 , Alexander Traxl 2 , Cécile Philippe 3 , Maria Weber 1 , Stephanie Häusler 4 , Bruno Stieger 4 , Walter Jäger 5 , Severin Mairinger 2 , Thomas Wanek 2 , Marcus Hacker 3 , Markus Zeitlinger 1 , Oliver Langer 1, 2, 3
Affiliation
|
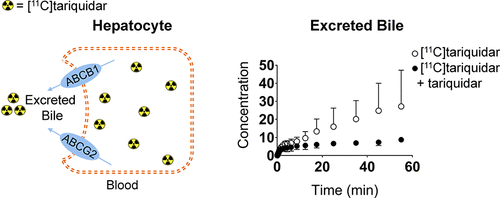
P-Glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) in the canalicular membrane of hepatocytes mediate the biliary excretion of drugs and drug metabolites. To measure hepatic ABCB1 and ABCG2 activity, we performed positron emission tomography (PET) scans with the ABCB1/ABCG2 substrate [11C]tariquidar in healthy volunteers and wild-type, Abcb1a/b(-/-), Abcg2(-/-), and Abcb1a/b(-/-)Abcg2(-/-) mice without and with coadministration of unlabeled tariquidar. PET data were analyzed with a three-compartment pharmacokinetic model. [11C]Tariquidar underwent hepatobiliary excretion in both humans and mice, and tariquidar coadministration caused a significant reduction in the rate constant for the transfer of radioactivity from the liver into bile (by -74% in humans and by -62% in wild-type mice), suggesting inhibition of canalicular efflux transporter activity. Radio-thin-layer chromatography analysis revealed that the majority of radioactivity (>87%) in the mouse liver and bile was composed of unmetabolized [11C]tariquidar. PET data in transporter knockout mice revealed that both ABCB1 and ABCG2 mediated biliary excretion of [11C]tariquidar. In vitro experiments indicated that tariquidar is not a substrate of major hepatic basolateral uptake transporters (SLCO1B1, SLCO1B3, SLCO2B1, SLC22A1, and SLC22A3). Our data suggest that [11C]tariquidar can be used to measure hepatic canalicular ABCB1/ABCG2 transport activity without a confounding effect of uptake transporters.
中文翻译:

[11C] Tariquidar和PET在人和小鼠中测量肝ABCB1和ABCG2转运活性。
肝细胞小管膜中的P-糖蛋白(ABCB1)和乳腺癌抗性蛋白(ABCG2)介导药物和药物代谢物的胆汁排泄。为了测量肝ABCB1和ABCG2的活性,我们在健康志愿者和野生型,Abcb1a / b(-/-),Abcg2(-/-)中使用ABCB1 / ABCG2底物[11C] qui参进行了正电子发射断层扫描(PET)扫描。 ,以及未和同时服用未标记的tariquidar的Abcb1a / b(-/-)Abcg2(-/-)小鼠。用三室药代动力学模型分析PET数据。[11C] Tariquidar在人和小鼠中都经历了肝胆排泄,并且tariquidar共同给药导致放射性从肝脏转移到胆汁的速率常数显着降低(在人类中为-74%,在野生型中为-62%老鼠),提示抑制小管外排转运蛋白活性。放射性薄层色谱分析表明,小鼠肝脏和胆汁中的大多数放射性(> 87%)由未代谢的[11C] qui藜芦醇组成。转运蛋白敲除小鼠的PET数据显示,ABCB1和ABCG2均介导[11C] tariquidar的胆汁排泄。体外实验表明,tariquidar不是主要肝基底外侧摄取转运蛋白(SLCO1B1,SLCO1B3,SLCO2B1,SLC22A1和SLC22A3)的底物。我们的数据表明,[11C] ari藜芦可用于测量肝小管ABCB1 / ABCG2的转运活性,而不会引起摄取转运体的混淆。小鼠肝脏和胆汁中有87%是由未代谢的[11C]塔里奇达(taariquidar)组成。转运蛋白敲除小鼠的PET数据显示,ABCB1和ABCG2均介导[11C] tariquidar的胆汁排泄。体外实验表明,tariquidar不是主要肝基底外侧摄取转运蛋白(SLCO1B1,SLCO1B3,SLCO2B1,SLC22A1和SLC22A3)的底物。我们的数据表明,[11C] qui藜芦可用于测量肝小管ABCB1 / ABCG2的转运活性,而不会引起摄取转运体的混淆。小鼠肝脏和胆汁中有87%是由未代谢的[11C]塔里奇达(taariquidar)组成。转运蛋白敲除小鼠的PET数据显示,ABCB1和ABCG2均介导[11C] tariquidar的胆汁排泄。体外实验表明,tariquidar不是主要肝基底外侧摄取转运蛋白(SLCO1B1,SLCO1B3,SLCO2B1,SLC22A1和SLC22A3)的底物。我们的数据表明,[11C] ari藜芦可用于测量肝小管ABCB1 / ABCG2的转运活性,而不会引起摄取转运体的混淆。
更新日期:2019-12-18
中文翻译:

[11C] Tariquidar和PET在人和小鼠中测量肝ABCB1和ABCG2转运活性。
肝细胞小管膜中的P-糖蛋白(ABCB1)和乳腺癌抗性蛋白(ABCG2)介导药物和药物代谢物的胆汁排泄。为了测量肝ABCB1和ABCG2的活性,我们在健康志愿者和野生型,Abcb1a / b(-/-),Abcg2(-/-)中使用ABCB1 / ABCG2底物[11C] qui参进行了正电子发射断层扫描(PET)扫描。 ,以及未和同时服用未标记的tariquidar的Abcb1a / b(-/-)Abcg2(-/-)小鼠。用三室药代动力学模型分析PET数据。[11C] Tariquidar在人和小鼠中都经历了肝胆排泄,并且tariquidar共同给药导致放射性从肝脏转移到胆汁的速率常数显着降低(在人类中为-74%,在野生型中为-62%老鼠),提示抑制小管外排转运蛋白活性。放射性薄层色谱分析表明,小鼠肝脏和胆汁中的大多数放射性(> 87%)由未代谢的[11C] qui藜芦醇组成。转运蛋白敲除小鼠的PET数据显示,ABCB1和ABCG2均介导[11C] tariquidar的胆汁排泄。体外实验表明,tariquidar不是主要肝基底外侧摄取转运蛋白(SLCO1B1,SLCO1B3,SLCO2B1,SLC22A1和SLC22A3)的底物。我们的数据表明,[11C] ari藜芦可用于测量肝小管ABCB1 / ABCG2的转运活性,而不会引起摄取转运体的混淆。小鼠肝脏和胆汁中有87%是由未代谢的[11C]塔里奇达(taariquidar)组成。转运蛋白敲除小鼠的PET数据显示,ABCB1和ABCG2均介导[11C] tariquidar的胆汁排泄。体外实验表明,tariquidar不是主要肝基底外侧摄取转运蛋白(SLCO1B1,SLCO1B3,SLCO2B1,SLC22A1和SLC22A3)的底物。我们的数据表明,[11C] qui藜芦可用于测量肝小管ABCB1 / ABCG2的转运活性,而不会引起摄取转运体的混淆。小鼠肝脏和胆汁中有87%是由未代谢的[11C]塔里奇达(taariquidar)组成。转运蛋白敲除小鼠的PET数据显示,ABCB1和ABCG2均介导[11C] tariquidar的胆汁排泄。体外实验表明,tariquidar不是主要肝基底外侧摄取转运蛋白(SLCO1B1,SLCO1B3,SLCO2B1,SLC22A1和SLC22A3)的底物。我们的数据表明,[11C] ari藜芦可用于测量肝小管ABCB1 / ABCG2的转运活性,而不会引起摄取转运体的混淆。



























 京公网安备 11010802027423号
京公网安备 11010802027423号