当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Combined Experimental and Molecular Simulation Study of Insulin-Chitosan Complexation Driven by Electrostatic Interactions.
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-01-06 , DOI: 10.1021/acs.jcim.9b00814 Cecilia Prudkin-Silva 1 , Oscar E Pérez 1 , Karina D Martínez 2 , Fernando L Barroso da Silva 3, 4
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-01-06 , DOI: 10.1021/acs.jcim.9b00814 Cecilia Prudkin-Silva 1 , Oscar E Pérez 1 , Karina D Martínez 2 , Fernando L Barroso da Silva 3, 4
Affiliation
|
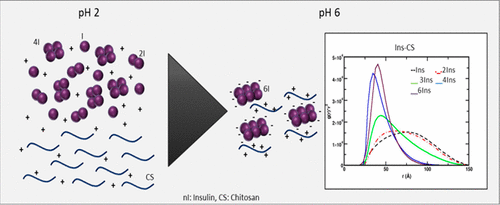
Protein-polysaccharide complexes constructed via self-assembly methods are often used to develop novel biomaterials for a wide range of applications in biomedicine, food, and biotechnology. The objective of this work was to investigate theoretically and to demonstrate via constant-pH Monte Carlo simulations that the complexation phenomenon between insulin (INS) and the cationic polyelectrolyte chitosan (CS) is mainly driven by an electrostatic mechanism. Experimental results obtained from FTIR spectra and ζ-potential determinations allowed us to complement the conclusions. The characteristic absorption bands for the complexes could be assigned to a combination of signals from CS amide I and INS amide II. The second peak corresponds to the interaction between the polymer and the protein at the level of amide II. INS-CS complexation processes not expected when INS is in its monomeric form, but for both tetrameric and hexameric forms, incipient complexation due to charge regulation mechanism took place at pH 5. The complexation range was observed to be 5.5 < pH < 6.5. In general, when the number of INS units increases in the simulation process, the solution pH at which the complexation can occur shifts toward acidic conditions. CS's chain interacts more efficiently, i.e. in a wider pH range, with INS aggregates formed by the highest monomer number. The charge regulation mechanism can be considered as a previous phase toward complexation (incipient complexation) caused by weak interactions of a Coulombic nature.
中文翻译:

静电相互作用驱动胰岛素-壳聚糖络合的联合实验和分子模拟研究。
通过自组装方法构建的蛋白质-多糖复合物通常用于开发新颖的生物材料,从而广泛应用于生物医学,食品和生物技术中。这项工作的目的是从理论上进行研究,并通过恒定pH值的蒙特卡洛模拟证明胰岛素(INS)和阳离子聚电解质壳聚糖(CS)之间的络合现象主要是由静电机制驱动的。从FTIR光谱和ζ电位测定获得的实验结果使我们可以补充这些结论。络合物的特征吸收带可以分配给CS酰胺I和INS酰胺II信号的组合。第二个峰对应于酰胺II水平上聚合物与蛋白质之间的相互作用。当INS为单体形式时,预计不会发生INS-CS络合过程,但对于四聚体和六聚体形式,由于电荷调节机制而在pH 5时发生了初始络合。观察到的络合范围为5.5 <pH <6.5。通常,当在模拟过程中增加INS单元的数量时,可能发生络合的溶液pH向酸性条件转移。CS的链更有效地相互作用,即在更宽的pH范围内,与由最高单体数形成的INS聚集体相互作用。电荷调节机制可以看作是由于库仑性质的弱相互作用而导致的络合(初期络合)的前一个阶段。由于电荷调节机制,初始络合发生在pH5。观察到络合范围为5.5 <pH <6.5。通常,当在模拟过程中增加INS单元的数量时,可能发生络合的溶液pH向酸性条件转移。CS的链更有效地相互作用,即在更宽的pH范围内,与由最高单体数形成的INS聚集体相互作用。电荷调节机制可以看作是由于库仑性质的弱相互作用而导致的络合(初期络合)的前一个阶段。由于电荷调节机制,初始络合发生在pH5。观察到络合范围为5.5 <pH <6.5。通常,当在模拟过程中增加INS单元的数量时,可能发生络合的溶液pH向酸性条件转移。CS的链更有效地相互作用,即在更宽的pH范围内,与由最高单体数形成的INS聚集体相互作用。电荷调节机制可以看作是由于库仑性质的弱相互作用而导致的络合(初期络合)的前一个阶段。S链更有效地相互作用,即在更宽的pH范围内,与由最高单体数形成的INS聚集体相互作用。电荷调节机制可以看作是由于库仑性质的弱相互作用而导致的络合(初期络合)的前一个阶段。S链更有效地相互作用,即在更宽的pH范围内,与由最高单体数形成的INS聚集体相互作用。电荷调节机制可以看作是由于库仑性质的弱相互作用而导致的络合(初期络合)的前一个阶段。
更新日期:2020-01-06
中文翻译:

静电相互作用驱动胰岛素-壳聚糖络合的联合实验和分子模拟研究。
通过自组装方法构建的蛋白质-多糖复合物通常用于开发新颖的生物材料,从而广泛应用于生物医学,食品和生物技术中。这项工作的目的是从理论上进行研究,并通过恒定pH值的蒙特卡洛模拟证明胰岛素(INS)和阳离子聚电解质壳聚糖(CS)之间的络合现象主要是由静电机制驱动的。从FTIR光谱和ζ电位测定获得的实验结果使我们可以补充这些结论。络合物的特征吸收带可以分配给CS酰胺I和INS酰胺II信号的组合。第二个峰对应于酰胺II水平上聚合物与蛋白质之间的相互作用。当INS为单体形式时,预计不会发生INS-CS络合过程,但对于四聚体和六聚体形式,由于电荷调节机制而在pH 5时发生了初始络合。观察到的络合范围为5.5 <pH <6.5。通常,当在模拟过程中增加INS单元的数量时,可能发生络合的溶液pH向酸性条件转移。CS的链更有效地相互作用,即在更宽的pH范围内,与由最高单体数形成的INS聚集体相互作用。电荷调节机制可以看作是由于库仑性质的弱相互作用而导致的络合(初期络合)的前一个阶段。由于电荷调节机制,初始络合发生在pH5。观察到络合范围为5.5 <pH <6.5。通常,当在模拟过程中增加INS单元的数量时,可能发生络合的溶液pH向酸性条件转移。CS的链更有效地相互作用,即在更宽的pH范围内,与由最高单体数形成的INS聚集体相互作用。电荷调节机制可以看作是由于库仑性质的弱相互作用而导致的络合(初期络合)的前一个阶段。由于电荷调节机制,初始络合发生在pH5。观察到络合范围为5.5 <pH <6.5。通常,当在模拟过程中增加INS单元的数量时,可能发生络合的溶液pH向酸性条件转移。CS的链更有效地相互作用,即在更宽的pH范围内,与由最高单体数形成的INS聚集体相互作用。电荷调节机制可以看作是由于库仑性质的弱相互作用而导致的络合(初期络合)的前一个阶段。S链更有效地相互作用,即在更宽的pH范围内,与由最高单体数形成的INS聚集体相互作用。电荷调节机制可以看作是由于库仑性质的弱相互作用而导致的络合(初期络合)的前一个阶段。S链更有效地相互作用,即在更宽的pH范围内,与由最高单体数形成的INS聚集体相互作用。电荷调节机制可以看作是由于库仑性质的弱相互作用而导致的络合(初期络合)的前一个阶段。











































 京公网安备 11010802027423号
京公网安备 11010802027423号