当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enzymatic Ligation of a Pore Blocker Toxin and a Gating Modifier Toxin: Creating Double-Knotted Peptides with Improved Sodium Channel NaV1.7 Inhibition.
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2019-12-16 , DOI: 10.1021/acs.bioconjchem.9b00744 Hue N T Tran 1 , Poanna Tran 1 , Jennifer R Deuis 1 , Akello J Agwa 1 , Alan H Zhang 2 , Irina Vetter 1, 3 , Christina I Schroeder 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2019-12-16 , DOI: 10.1021/acs.bioconjchem.9b00744 Hue N T Tran 1 , Poanna Tran 1 , Jennifer R Deuis 1 , Akello J Agwa 1 , Alan H Zhang 2 , Irina Vetter 1, 3 , Christina I Schroeder 1
Affiliation
|
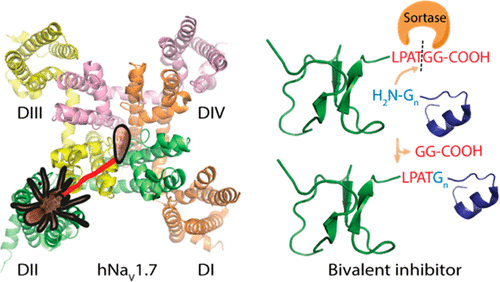
Disulfide-rich animal venom peptides targeting either the voltage-sensing domain or the pore domain of voltage-gated sodium channel 1.7 (NaV1.7) have been widely studied as drug leads and pharmacological probes for the treatment of chronic pain. However, despite intensive research efforts, the full potential of NaV1.7 as a therapeutic target is yet to be realized. In this study, using evolved sortase A, we enzymatically ligated two known NaV1.7 inhibitors-PaurTx3, a spider-derived peptide toxin that modifies the gating mechanism of the channel through interaction with the voltage-sensing domain, and KIIIA, a small cone snail-derived peptide inhibitor of the pore domain-with the aim of creating a bivalent inhibitor which could interact simultaneously with two noncompeting binding sites. Using electrophysiology, we determined the activity at NaV1.7, and to maximize potency, we systematically evaluated the optimal linker length, which was nine amino acids. Our optimized synthetic bivalent peptide showed improved channel affinity and potency at NaV1.7 compared to either PaurTx3 or KIIIA individually. This work shows that novel and improved NaV1.7 inhibitors can be designed by combining a pore blocker toxin and a gating modifier toxin to confer desired pharmacological properties from both the voltage sensing domain and the pore domain.
中文翻译:

酶促连接毛孔阻断剂毒素和门控修饰剂毒素:创建具有改进的钠通道NaV1.7抑制作用的双结肽。
靶向电压门控钠通道1.7(NaV1.7)的电压感应域或孔域的富含二硫键的动物毒肽已被广泛用作治疗慢性疼痛的药物引线和药理探针。然而,尽管进行了深入的研究,但NaV1.7作为治疗靶标的全部潜力尚未实现。在这项研究中,我们使用进化的分选酶A,通过酶法连接了两种已知的NaV1.7抑制剂-PaurTx3,这是一种蜘蛛衍生的肽毒素,可通过与电压感应域相互作用来修饰通道的门控机制,而KIIIA是一个小锥蜗牛衍生的孔结构域肽抑制剂-目的是创建可以与两个非竞争性结合位点同时相互作用的二价抑制剂。利用电生理学,我们确定了NaV1.7的活性,为了最大化效价,我们系统地评估了最佳接头长度,即9个氨基酸。与单独的PaurTx3或KIIIA相比,我们优化的合成二价肽在NaV1.7处显示出更高的通道亲和力和效能。这项工作表明,可以通过组合毛孔阻断剂毒素和门控修饰剂毒素来设计新颖且改良的NaV1.7抑制剂,以从电压感应域和孔域中赋予所需的药理特性。
更新日期:2019-12-17
中文翻译:

酶促连接毛孔阻断剂毒素和门控修饰剂毒素:创建具有改进的钠通道NaV1.7抑制作用的双结肽。
靶向电压门控钠通道1.7(NaV1.7)的电压感应域或孔域的富含二硫键的动物毒肽已被广泛用作治疗慢性疼痛的药物引线和药理探针。然而,尽管进行了深入的研究,但NaV1.7作为治疗靶标的全部潜力尚未实现。在这项研究中,我们使用进化的分选酶A,通过酶法连接了两种已知的NaV1.7抑制剂-PaurTx3,这是一种蜘蛛衍生的肽毒素,可通过与电压感应域相互作用来修饰通道的门控机制,而KIIIA是一个小锥蜗牛衍生的孔结构域肽抑制剂-目的是创建可以与两个非竞争性结合位点同时相互作用的二价抑制剂。利用电生理学,我们确定了NaV1.7的活性,为了最大化效价,我们系统地评估了最佳接头长度,即9个氨基酸。与单独的PaurTx3或KIIIA相比,我们优化的合成二价肽在NaV1.7处显示出更高的通道亲和力和效能。这项工作表明,可以通过组合毛孔阻断剂毒素和门控修饰剂毒素来设计新颖且改良的NaV1.7抑制剂,以从电压感应域和孔域中赋予所需的药理特性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号