Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Maternal prescribed opioid analgesic use during pregnancy and associations with adverse birth outcomes: A population-based study.
PLOS Medicine ( IF 10.5 ) Pub Date : 2019-12-02 , DOI: 10.1371/journal.pmed.1002980 Ayesha C Sujan 1 , Patrick D Quinn 2 , Martin E Rickert 1 , Kelsey K Wiggs 1 , Paul Lichtenstein 3 , Henrik Larsson 3, 4 , Catarina Almqvist 3, 5 , A Sara Öberg 3, 6 , Brian M D'Onofrio 1, 3
PLOS Medicine ( IF 10.5 ) Pub Date : 2019-12-02 , DOI: 10.1371/journal.pmed.1002980 Ayesha C Sujan 1 , Patrick D Quinn 2 , Martin E Rickert 1 , Kelsey K Wiggs 1 , Paul Lichtenstein 3 , Henrik Larsson 3, 4 , Catarina Almqvist 3, 5 , A Sara Öberg 3, 6 , Brian M D'Onofrio 1, 3
Affiliation
|
BACKGROUND
Published research on prescribed opioid analgesic (POA) use during pregnancy and birth outcomes is limited in scope and has not adequately adjusted for potential confounding factors. To help address these gaps, we estimated associations between maternal POAs during pregnancy and two adverse birth outcomes using a large population-based dataset, multiple definitions of POA exposure, and several methods to evaluate the influence of both measured and unmeasured confounding factors.
METHODS AND FINDINGS
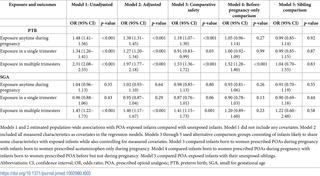
We obtained data by linking information from several Swedish registers and conducted a retrospective cohort study on a population-based sample of 620,458 Swedish births occurring between 2007 and 2013 (48.6% female; 44.4% firstborn). We evaluated associations between prenatal POA exposure and risk for preterm birth (PTB; <37 gestational weeks) and small for gestational age (SGA; birth weight 2 standard deviations below the expected weight for gestational age or lower). We evaluated the influence of confounding by adjusting for a wide range of measured covariates while comparing exposed and unexposed infants. Additionally, we adjusted for unmeasured confounding factors by using several advanced epidemiological designs. Infants exposed to POAs anytime during pregnancy were at increased risk for PTB compared with unexposed infants (6.4% exposed versus 4.4% unexposed; adjusted odds ratio [OR] = 1.38, 95% confidence interval [CI] 1.31-1.45, p < 0.001). This association was attenuated when we compared POA-exposed infants with acetaminophen-exposed infants (OR = 1.18, 95% CI 1.07-1.30, p < 0.001), infants born to women who used POAs before pregnancy only (OR = 1.05, 95% CI 0.96-1.14, p = 0.27), and unexposed siblings (OR = 0.99, 95% CI 0.85-1.14, p = 0.92). We also evaluated associations with short-term versus persistent POA use during pregnancy and observed a similar pattern of results, although the magnitudes of associations with persistent exposure were larger than associations with any use or short-term use. Although short-term use was not associated with SGA (adjusted ORsingle-trimester = 0.95, 95% CI 0.87-1.04, p = 0.29), persistent use was associated with increased risk for SGA (adjusted ORmultiple-trimester = 1.40, 95% CI 1.17-1.67, p < 0.001) compared with unexposed infants. The association with persistent exposure was attenuated when we used alternative comparison groups (e.g., sibling comparison OR = 1.22, 95% CI 0.60-2.48, p = 0.58). Of note, our study had limitations, including potential bias from exposure misclassification, an inability to adjust for all sources of confounding, and uncertainty regarding generalizability to countries outside of Sweden.
CONCLUSIONS
Our results suggested that observed associations between POA use during pregnancy and risk of PTB and SGA were largely due to unmeasured confounding factors, although we could not rule out small independent associations, particularly for persistent POA use during pregnancy.
中文翻译:

孕妇在怀孕期间使用阿片类镇痛剂以及与不良出生结局的关联:一项基于人群的研究。
背景技术已公开的关于在怀孕和分娩过程中使用阿片类镇痛药(POA)的研究范围有限,并且尚未针对潜在的混杂因素进行适当调整。为了解决这些差距,我们使用大量的基于人口的数据集,POA暴露的多种定义以及几种评估测量和未测量混杂因素影响的方法,估计了孕妇在怀孕期间的POA与两个不良分娩结局之间的关联。方法和研究结果我们通过链接来自多个瑞典登记处的信息获得了数据,并对2007年至2013年间以人口为基础的620458例瑞典出生人口进行了回顾性队列研究(女性为48.6%;第一胎为44.4%)。我们评估了产前POA暴露与早产风险(PTB; < 胎龄37周),胎龄较小(SGA;出生体重2个标准差,小于胎龄或更轻的预期体重)。我们在比较暴露和未暴露的婴儿时,通过调整各种测量的协变量来评估混淆的影响。此外,我们通过使用几种先进的流行病学设计调整了无法衡量的混杂因素。与未暴露的婴儿相比,怀孕期间随时暴露于POA的婴儿患PTB的风险增加(6.4%暴露与未暴露的4.4%;调整后的优势比[OR] = 1.38,95%的置信区间[CI] 1.31-1.45,p <0.001) 。当我们将暴露于POA的婴儿与对乙酰氨基酚暴露的婴儿(OR = 1.18,95%CI 1.07-1.30,p <0.001),仅在怀孕前使用过POA的女性所生的婴儿(OR = 1.05,95%CI 0.96-1.14,p = 0.27)和未暴露的兄弟姐妹(OR = 0.99,95%CI 0.85-1.14,p = 0.92)。我们还评估了妊娠期间短期和长期使用POA的关联,并观察到了相似的结果模式,尽管持续暴露的关联的规模大于任何使用或短期使用的关联。尽管短期使用与SGA无关(调整后的单孕期= 0.95,95%CI 0.87-1.04,p = 0.29),但长期使用与SGA的风险增加相关(调整后的多期妊娠= 1.40,95%CI与未暴露的婴儿相比,为1.17-1.67,p <0.001)。当我们使用替代比较组时(例如,同级比较OR = 1.22,95%CI 0.60-2.48,p = 0.58),与持续性暴露的关联减弱。值得注意的是,我们的研究有局限性,包括暴露分类错误的潜在偏见,无法对所有混杂因素进行调整以及对瑞典以外国家/地区可推广性的不确定性。结论我们的结果表明,观察到的妊娠期POA使用与PTB和SGA风险之间的关联主要是由于无法测量的混杂因素,尽管我们不能排除小的独立关联,特别是妊娠期持续使用POA。
更新日期:2020-01-14
中文翻译:

孕妇在怀孕期间使用阿片类镇痛剂以及与不良出生结局的关联:一项基于人群的研究。
背景技术已公开的关于在怀孕和分娩过程中使用阿片类镇痛药(POA)的研究范围有限,并且尚未针对潜在的混杂因素进行适当调整。为了解决这些差距,我们使用大量的基于人口的数据集,POA暴露的多种定义以及几种评估测量和未测量混杂因素影响的方法,估计了孕妇在怀孕期间的POA与两个不良分娩结局之间的关联。方法和研究结果我们通过链接来自多个瑞典登记处的信息获得了数据,并对2007年至2013年间以人口为基础的620458例瑞典出生人口进行了回顾性队列研究(女性为48.6%;第一胎为44.4%)。我们评估了产前POA暴露与早产风险(PTB; < 胎龄37周),胎龄较小(SGA;出生体重2个标准差,小于胎龄或更轻的预期体重)。我们在比较暴露和未暴露的婴儿时,通过调整各种测量的协变量来评估混淆的影响。此外,我们通过使用几种先进的流行病学设计调整了无法衡量的混杂因素。与未暴露的婴儿相比,怀孕期间随时暴露于POA的婴儿患PTB的风险增加(6.4%暴露与未暴露的4.4%;调整后的优势比[OR] = 1.38,95%的置信区间[CI] 1.31-1.45,p <0.001) 。当我们将暴露于POA的婴儿与对乙酰氨基酚暴露的婴儿(OR = 1.18,95%CI 1.07-1.30,p <0.001),仅在怀孕前使用过POA的女性所生的婴儿(OR = 1.05,95%CI 0.96-1.14,p = 0.27)和未暴露的兄弟姐妹(OR = 0.99,95%CI 0.85-1.14,p = 0.92)。我们还评估了妊娠期间短期和长期使用POA的关联,并观察到了相似的结果模式,尽管持续暴露的关联的规模大于任何使用或短期使用的关联。尽管短期使用与SGA无关(调整后的单孕期= 0.95,95%CI 0.87-1.04,p = 0.29),但长期使用与SGA的风险增加相关(调整后的多期妊娠= 1.40,95%CI与未暴露的婴儿相比,为1.17-1.67,p <0.001)。当我们使用替代比较组时(例如,同级比较OR = 1.22,95%CI 0.60-2.48,p = 0.58),与持续性暴露的关联减弱。值得注意的是,我们的研究有局限性,包括暴露分类错误的潜在偏见,无法对所有混杂因素进行调整以及对瑞典以外国家/地区可推广性的不确定性。结论我们的结果表明,观察到的妊娠期POA使用与PTB和SGA风险之间的关联主要是由于无法测量的混杂因素,尽管我们不能排除小的独立关联,特别是妊娠期持续使用POA。











































 京公网安备 11010802027423号
京公网安备 11010802027423号