The Pharmacogenomics Journal ( IF 2.9 ) Pub Date : 2019-12-03 , DOI: 10.1038/s41397-019-0134-9 Akira Onishi 1 , Shigeo Kamitsuji 2 , Miwa Nishida 3 , Yuko Uemura 4 , Miho Takahashi 4 , Toshiharu Saito 4 , Yuichiro Yoshida 5 , Masaki Kobayashi 5 , Mizuho Kawate 5 , Keisuke Nishimura 6 , Kenta Misaki 7 , Yumiko Nobuhara 8 , Takashi Nakazawa 8 , Saori Hatachi 3, 9 , Goh Tsuji 3 , Akio Morinobu 1 , Shunichi Kumagai 3, 4
|
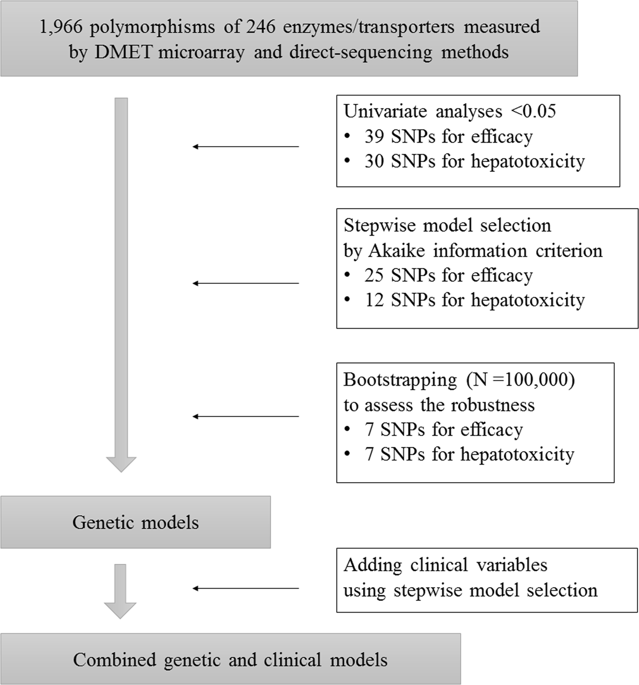
The objective of the study is to develop genetic and clinical prediction models for the efficacy and hepatotoxicity of methotrexate (MTX) in patients with rheumatoid arthritis (RA). Among RA patients treated with MTX, 1966 polymorphisms of 246 enzymes/transporters relevant to pharmacokinetics and pharmacodynamics were measured by the Drug Metabolism Enzymes and Transporters (DMET) microarray and direct sequencing, and clinical variables at baseline were collected. For efficacy, response criteria of the European League Against Rheumatism were used to classify patients as responders or non-responders. Hepatotoxicity was defined as elevations of aspartate aminotransferase or alanine aminotransferase ≥1.5 times the reference range upper limit. Among 166 patients, a genetic prediction model for efficacy using seven polymorphisms showed the area under the receiver operating characteristic curve (AUC) was 0.822, with 74.3% sensitivity and 76.8% specificity. A combined genetic and clinical model indicated the AUC was 0.844, with 81.5% sensitivity and 76.9% specificity. By incorporating clinical variables into the genetic model, the overall category-free net reclassification improvement (NRI) was 0.663 (P < 0.0001) and the overall integrated discrimination improvement (IDI) was 0.083 (P = 0.0009). For hepatotoxicity, a genetic prediction model using seven polymorphisms showed the AUC was 0.783 with 70.0% sensitivity and 80.0% specificity, while the combined model indicated the AUC was 0.906 with 85.1% sensitivity and 87.8% specificity (overall category-free NRI: 1.002, P < 0.0001; overall IDI: 0.254, P < 0.0001). Our genetic and clinical models demonstrated moderate diagnostic accuracy for MTX efficacy and high accuracy for hepatotoxicity. These findings should, however, be validated and interpreted with a caution until external validation.
中文翻译:

甲氨蝶呤对类风湿关节炎患者的疗效和肝毒性的遗传和临床预测模型:一项多中心队列研究。
这项研究的目的是为甲氨蝶呤(MTX)在类风湿关节炎(RA)患者中的疗效和肝毒性开发遗传和临床预测模型。在接受MTX治疗的RA患者中,通过药物代谢酶和转运蛋白(DMET)微阵列和直接测序法测量了1966年与药代动力学和药效学相关的246种酶/转运蛋白的多态性,并收集了基线的临床变量。为了疗效,使用欧洲风湿病联盟的缓解标准将患者分类为缓解者或非缓解者。肝毒性定义为天冬氨酸转氨酶或丙氨酸转氨酶的升高≥参考范围上限的1.5倍。在166例患者中,使用七个多态性的功效遗传预测模型显示,受体工作特征曲线(AUC)下的面积为0.822,灵敏度为74.3%,特异性为76.8%。遗传和临床相结合的模型表明,AUC为0.844,敏感性为81.5%,特异性为76.9%。通过将临床变量纳入遗传模型,总的无类别净重分类改进(NRI)为0.663(P <0.0001),整体综合歧视改善(IDI)为0.083(P = 0.0009)。对于肝毒性,使用七个多态性的遗传预测模型显示AUC为0.783,敏感性为70.0%,特异性为80.0%,而组合模型显示AUC为0.906,敏感性为85.1%,特异性为87.8%(总体无类别NRI:1.002,P <0.0001;总体IDI:0.254,P <0.0001)。我们的遗传和临床模型证明了MTX疗效的中等诊断准确度和肝毒性的准确度。但是,在进行外部验证之前,应谨慎验证和解释这些发现。











































 京公网安备 11010802027423号
京公网安备 11010802027423号