当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Insights into Interaction Mechanisms of Alternative Piperazine-urea YEATS Domain Binders in MLLT1.
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2019-11-25 , DOI: 10.1021/acsmedchemlett.9b00460 Xiaomin Ni 1, 2 , David Heidenreich 1, 2 , Thomas Christott 3 , James Bennett 3 , Moses Moustakim 3 , Paul E Brennan 1, 2, 3 , Oleg Fedorov 3 , Stefan Knapp 1, 2 , Apirat Chaikuad 1, 2
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2019-11-25 , DOI: 10.1021/acsmedchemlett.9b00460 Xiaomin Ni 1, 2 , David Heidenreich 1, 2 , Thomas Christott 3 , James Bennett 3 , Moses Moustakim 3 , Paul E Brennan 1, 2, 3 , Oleg Fedorov 3 , Stefan Knapp 1, 2 , Apirat Chaikuad 1, 2
Affiliation
|
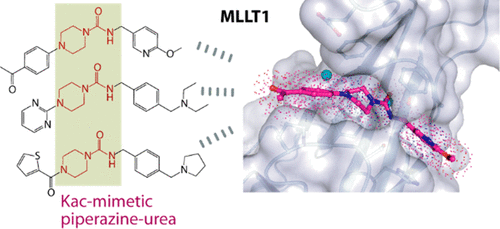
YEATS-domain-containing MLLT1 is an acetyl/acyl-lysine reader domain, which is structurally distinct from well-studied bromodomains and has been strongly associated in development of cancer. Here, we characterized piperazine-urea derivatives as an acetyl/acyl-lysine mimetic moiety for MLLT1. Crystal structures revealed distinct interaction mechanisms of this chemotype compared to the recently described benzimidazole-amide based inhibitors, exploiting different binding pockets within the protein. Thus, the piperazine-urea scaffold offers an alternative strategy for targeting the YEATS domain family.
中文翻译:

MLLT1中替代哌嗪-脲YEATS域结合剂相互作用机理的结构性见解。
包含YEATS结构域的MLLT1是一个乙酰基/酰基赖氨酸阅读器结构域,其结构与研究透彻的溴结构域不同,并且与癌症的发展密切相关。在这里,我们将哌嗪-脲衍生物表征为MLLT1的乙酰基/酰基赖氨酸模拟部分。与最近描述的基于苯并咪唑-酰胺的抑制剂相比,晶体结构揭示了该化学型的独特相互作用机制,从而利用了蛋白质内的不同结合口袋。因此,哌嗪-脲支架为靶向YEATS域家族提供了另一种策略。
更新日期:2019-12-03
中文翻译:

MLLT1中替代哌嗪-脲YEATS域结合剂相互作用机理的结构性见解。
包含YEATS结构域的MLLT1是一个乙酰基/酰基赖氨酸阅读器结构域,其结构与研究透彻的溴结构域不同,并且与癌症的发展密切相关。在这里,我们将哌嗪-脲衍生物表征为MLLT1的乙酰基/酰基赖氨酸模拟部分。与最近描述的基于苯并咪唑-酰胺的抑制剂相比,晶体结构揭示了该化学型的独特相互作用机制,从而利用了蛋白质内的不同结合口袋。因此,哌嗪-脲支架为靶向YEATS域家族提供了另一种策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号