当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
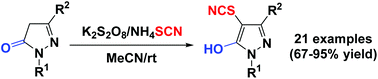
K2S2O8-promoted direct thiocyanation of pyrazolin-5-ones with ammonium thiocyanate at room temperature
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2019/12/03 , DOI: 10.1039/c9qo01174a Xiaokang Mao 1, 2, 3, 4, 5 , Jiabin Ni 6, 7, 8, 9, 10 , Bin Xu 1, 2, 3, 4, 5 , Chunyong Ding 6, 7, 8, 9, 10
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2019/12/03 , DOI: 10.1039/c9qo01174a Xiaokang Mao 1, 2, 3, 4, 5 , Jiabin Ni 6, 7, 8, 9, 10 , Bin Xu 1, 2, 3, 4, 5 , Chunyong Ding 6, 7, 8, 9, 10
Affiliation
|
A facile and efficient approach for the direct thiocyanation of pyrazolin-5-ones via a radical mechanism has been established for the first time. A series of 4-thiocyanated 5-hydroxy-1H-pyrazoles were synthesized in fair to good yields by K2S2O8-promoted direct thiocyanation of pyrazolin-5-ones with NH4SCN at room temperature under simple and mild reaction conditions with atom- and step-economy and good functional group tolerance.
中文翻译:

室温下用硫氰酸铵K2S2O8促进吡唑啉-5-酮的直接硫氰化
首次建立了一种通过自由基机理将吡唑啉-5-酮直接硫氰酸化的简便有效方法。在室温下,通过简单,温和的反应,通过K 2 S 2 O 8促进吡唑啉-5-酮与NH 4 SCN的直接硫氰化反应,合成了一系列4-硫氰酸化的5-羟基-1 H-吡唑,并以中等至良好的收率合成。具有原子经济和阶梯经济以及良好的官能团耐受性的条件。
更新日期:2020-02-13
中文翻译:

室温下用硫氰酸铵K2S2O8促进吡唑啉-5-酮的直接硫氰化
首次建立了一种通过自由基机理将吡唑啉-5-酮直接硫氰酸化的简便有效方法。在室温下,通过简单,温和的反应,通过K 2 S 2 O 8促进吡唑啉-5-酮与NH 4 SCN的直接硫氰化反应,合成了一系列4-硫氰酸化的5-羟基-1 H-吡唑,并以中等至良好的收率合成。具有原子经济和阶梯经济以及良好的官能团耐受性的条件。



























 京公网安备 11010802027423号
京公网安备 11010802027423号