当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Restoration of myocellular copper-trafficking proteins and mitochondrial copper enzymes repairs cardiac function in rats with diabetes-evoked heart failure.
Metallomics ( IF 2.9 ) Pub Date : 2019-12-10 , DOI: 10.1039/c9mt00223e Shaoping Zhang 1 , Hong Liu , Greeshma Vazhoor Amarsingh , Carlos C H Cheung , Donghai Wu , Umayal Narayanan , Linda Zhang , Garth J S Cooper
Metallomics ( IF 2.9 ) Pub Date : 2019-12-10 , DOI: 10.1039/c9mt00223e Shaoping Zhang 1 , Hong Liu , Greeshma Vazhoor Amarsingh , Carlos C H Cheung , Donghai Wu , Umayal Narayanan , Linda Zhang , Garth J S Cooper
Affiliation
|
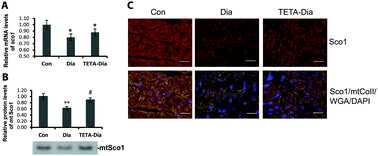
Diabetes impairs systemic copper regulation, and acts as a major independent risk factor for heart failure (HF) wherein mitochondrial dysfunction is a key pathogenic process. Here we asked whether diabetes might alter mitochondrial structure/function and thus impair cardiac performance by damaging myocellular pathways that mediate cell-copper homeostasis. We measured activity of major mitochondria-resident copper-enzymes cytochrome c oxidase (mt-Cco) and superoxide dismutase 1 (mt-Sod1); expression of three main mitochondrial copper-chaperones [Cco copper chaperone 17 (Cox17), Cox11, and mitochondria-resident copper chaperone for Sod1 (mt-Ccs)]; of copper-dependent Cco-assembly protein Sco1; and regulation of mitochondrial biogenesis, in left-ventricular (LV) tissue from groups of non-diabetic-control, untreated-diabetic, and divalent-copper-selective chelator-treated diabetic rats. Diabetes impaired LV pump function; ∼halved LV-copper levels; substantively decreased myocellular expression of copper chaperones, and enzymatic activity of mt-Cco and mt-Sod1. Divalent-copper chelation with triethylenetetramine improved cardiac pump function, restored levels of myocardial copper, the copper chaperones, and Sco1; and enzymatic activity of mt-Cco and mt-Sod1. Copper chelation also restored expression of the key mitochondrial biogenesis regulator, peroxisome-proliferator-activated receptor gamma co-activator-1α (Pgc-1α). This study shows for the first time that altered myocardial copper-trafficking is a key pathogenic process in diabetes-evoked HF. We also describe a novel therapeutic effect of divalent-copper-selective chelation, namely restoration of myocellular copper trafficking, which is thus revealed as a potentially tractable target for novel pharmacological intervention to improve cardiac function.
中文翻译:

心肌细胞铜转运蛋白和线粒体铜酶的恢复可修复糖尿病引起的心力衰竭大鼠的心功能。
糖尿病会损害全身的铜调节,并成为心力衰竭(HF)的主要独立危险因素,其中线粒体功能障碍是关键的致病过程。在这里,我们询问糖尿病是否会通过破坏介导细胞-铜稳态的介导的肌细胞通路而改变线粒体的结构/功能,从而损害心脏的功能。我们测量了主要的线粒体驻留铜酶细胞色素c氧化酶(mt-Cco)和超氧化物歧化酶1(mt-Sod1)的活性;三种主要的线粒体铜伴侣蛋白的表达[Sco1(mt-Ccs)的Cco铜伴侣蛋白17(Cox17),Cox11和线粒体驻留的铜伴侣蛋白];铜依赖性Cco装配蛋白Sco1的研究 非糖尿病对照组,未经治疗的糖尿病患者的左心室(LV)组织中线粒体生物发生的调控 和二价铜选择性螯合剂治疗的糖尿病大鼠。糖尿病LV泵功能受损;〜LV-铜水平减半;大大降低了铜伴侣蛋白的肌细胞表达以及mt-Cco和mt-Sod1的酶活性。三亚乙基四胺与二价铜螯合可改善心脏泵功能,恢复心肌铜,铜伴侣和Sco1的水平;和mt-Cco和mt-Sod1的酶活性。铜螯合还恢复了关键的线粒体生物发生调节因子过氧化物酶体增殖物激活受体γ共同激活因子1α(Pgc-1α)的表达。这项研究首次表明,改变心肌铜的运输是糖尿病诱发的心衰的关键致病过程。我们还描述了二价铜选择性螯合的新型治疗作用,
更新日期:2020-02-27
中文翻译:

心肌细胞铜转运蛋白和线粒体铜酶的恢复可修复糖尿病引起的心力衰竭大鼠的心功能。
糖尿病会损害全身的铜调节,并成为心力衰竭(HF)的主要独立危险因素,其中线粒体功能障碍是关键的致病过程。在这里,我们询问糖尿病是否会通过破坏介导细胞-铜稳态的介导的肌细胞通路而改变线粒体的结构/功能,从而损害心脏的功能。我们测量了主要的线粒体驻留铜酶细胞色素c氧化酶(mt-Cco)和超氧化物歧化酶1(mt-Sod1)的活性;三种主要的线粒体铜伴侣蛋白的表达[Sco1(mt-Ccs)的Cco铜伴侣蛋白17(Cox17),Cox11和线粒体驻留的铜伴侣蛋白];铜依赖性Cco装配蛋白Sco1的研究 非糖尿病对照组,未经治疗的糖尿病患者的左心室(LV)组织中线粒体生物发生的调控 和二价铜选择性螯合剂治疗的糖尿病大鼠。糖尿病LV泵功能受损;〜LV-铜水平减半;大大降低了铜伴侣蛋白的肌细胞表达以及mt-Cco和mt-Sod1的酶活性。三亚乙基四胺与二价铜螯合可改善心脏泵功能,恢复心肌铜,铜伴侣和Sco1的水平;和mt-Cco和mt-Sod1的酶活性。铜螯合还恢复了关键的线粒体生物发生调节因子过氧化物酶体增殖物激活受体γ共同激活因子1α(Pgc-1α)的表达。这项研究首次表明,改变心肌铜的运输是糖尿病诱发的心衰的关键致病过程。我们还描述了二价铜选择性螯合的新型治疗作用,











































 京公网安备 11010802027423号
京公网安备 11010802027423号