当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-catalyzed Sonogashira coupling reactions in γ-valerolactone-based ionic liquids.
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2019-12-03 , DOI: 10.3762/bjoc.15.284 László Orha 1, 2 , József M Tukacs 1 , László Kollár 3 , László T Mika 1
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2019-12-03 , DOI: 10.3762/bjoc.15.284 László Orha 1, 2 , József M Tukacs 1 , László Kollár 3 , László T Mika 1
Affiliation
|
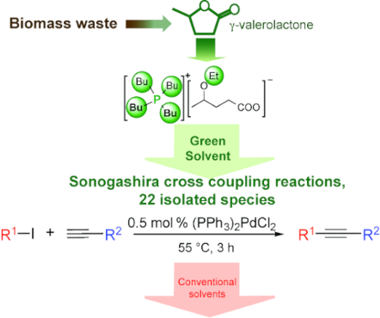
It was demonstrated that the γ-valerolactone-based ionic liquid, tetrabutylphosphonium 4-ethoxyvalerate as a partially bio-based solvent can be utilized as alternative reaction medium for copper- and auxiliary base-free Pd-catalyzed Sonogashira coupling reactions of aryl iodides and functionalized acetylenes under mild conditions. Twenty-two cross-coupling products were isolated with good to excellent yields (72-99%) and purity (>98%). These results represent an example which proves that biomass-derived safer solvents can be utilized efficiently in common, industrially important transformations exhibiting higher chemical and environmental efficiency.
中文翻译:

γ-戊内酯基离子液体中钯催化的Sonogashira偶联反应。
结果表明,基于γ-戊内酯的离子液体,4-乙氧基戊酸四丁基phosph作为部分生物基溶剂可以用作替代的反应介质,用于无铜和辅助的无钯钯催化的碘代芳基碘化物的Sonogashira偶联反应并进行功能化乙炔在温和的条件下。分离出二十二种交叉偶联产物,收率(72-99%)和纯度(> 98%)好至极好。这些结果代表了一个实例,证明了生物质衍生的更安全的溶剂可以有效地用于具有较高化学和环境效率的常见的,工业上重要的转化中。
更新日期:2019-12-03
中文翻译:

γ-戊内酯基离子液体中钯催化的Sonogashira偶联反应。
结果表明,基于γ-戊内酯的离子液体,4-乙氧基戊酸四丁基phosph作为部分生物基溶剂可以用作替代的反应介质,用于无铜和辅助的无钯钯催化的碘代芳基碘化物的Sonogashira偶联反应并进行功能化乙炔在温和的条件下。分离出二十二种交叉偶联产物,收率(72-99%)和纯度(> 98%)好至极好。这些结果代表了一个实例,证明了生物质衍生的更安全的溶剂可以有效地用于具有较高化学和环境效率的常见的,工业上重要的转化中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号