当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
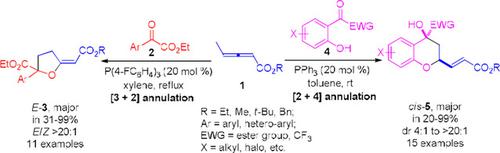
Phosphine‐Catalyzed [3+2] and [2+4] Annulations of γ‐Methyl Allenoates with Aryl α‐Keto Esters: Stereoselective Syntheses of Functionalized Tetrahydrofurans and 4‐Chromanols
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2019-12-10 , DOI: 10.1002/ajoc.201900635 Zifeng Qin 1 , Rongfang Liu 2 , Rong Zhou 2 , Ruifeng Li 2 , Zhengjie He 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2019-12-10 , DOI: 10.1002/ajoc.201900635 Zifeng Qin 1 , Rongfang Liu 2 , Rong Zhou 2 , Ruifeng Li 2 , Zhengjie He 1
Affiliation
|
Phosphine‐catalyzed [3+2] and [2+4] annulation reactions of γ‐methyl allenoates with aryl α‐keto esters are described. Under the catalysis of triarylphosphine, γ‐methyl allenoates participate in both the [3+2] annulation with aroylformates and [2+4] annulation with ortho‐hydroxy aroylformates smoothly to bring about functionalized tetrahydrofurans and 4‐chromanols, respectively, in moderate to excellent yields with high stereoselectivity. The reactions herein also represent rare successful examples of phosphine‐catalyzed allene−ketone transformations, and further expand the scope of ketones in allene chemistry under phosphine catalysis.
中文翻译:

膦催化的具有芳基α-酮基酯的γ-甲基丙烯酸酯的[3 + 2]和[2 + 4]环化:官能化的四氢呋喃和4-色酚的立体选择性合成
描述了磷化氢催化γ-甲基脲基酸酯与芳基α-酮酸酯的[3 + 2]和[2 + 4]环化反应。在三芳基膦的催化下,γ-甲基烯丙基酯顺利地以芳酰基甲酸酯参与[3 + 2]环合和以邻羟基芳酰基甲酸酯的[2 + 4]环合,分别适度地产生功能化的四氢呋喃和4-色酚。具有高立体选择性的优异收率。本文的反应也代表了膦催化的丙二烯-酮转化的罕见成功实例,并进一步扩大了膦催化下丙二烯化学中酮的范围。
更新日期:2019-12-11
中文翻译:

膦催化的具有芳基α-酮基酯的γ-甲基丙烯酸酯的[3 + 2]和[2 + 4]环化:官能化的四氢呋喃和4-色酚的立体选择性合成
描述了磷化氢催化γ-甲基脲基酸酯与芳基α-酮酸酯的[3 + 2]和[2 + 4]环化反应。在三芳基膦的催化下,γ-甲基烯丙基酯顺利地以芳酰基甲酸酯参与[3 + 2]环合和以邻羟基芳酰基甲酸酯的[2 + 4]环合,分别适度地产生功能化的四氢呋喃和4-色酚。具有高立体选择性的优异收率。本文的反应也代表了膦催化的丙二烯-酮转化的罕见成功实例,并进一步扩大了膦催化下丙二烯化学中酮的范围。











































 京公网安备 11010802027423号
京公网安备 11010802027423号