Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
mTOR-mediated metabolic reprogramming shapes distinct microglia functions in response to lipopolysaccharide and ATP.
Glia ( IF 5.4 ) Pub Date : 2019-12-03 , DOI: 10.1002/glia.23760 Yaling Hu 1 , Weihao Mai 1 , Lunhao Chen 1, 2 , Kelei Cao 1 , Bin Zhang 1 , Zhenjie Zhang 1 , Yijun Liu 1 , Huifang Lou 1 , Shumin Duan 1 , Zhihua Gao 1
Glia ( IF 5.4 ) Pub Date : 2019-12-03 , DOI: 10.1002/glia.23760 Yaling Hu 1 , Weihao Mai 1 , Lunhao Chen 1, 2 , Kelei Cao 1 , Bin Zhang 1 , Zhenjie Zhang 1 , Yijun Liu 1 , Huifang Lou 1 , Shumin Duan 1 , Zhihua Gao 1
Affiliation
|
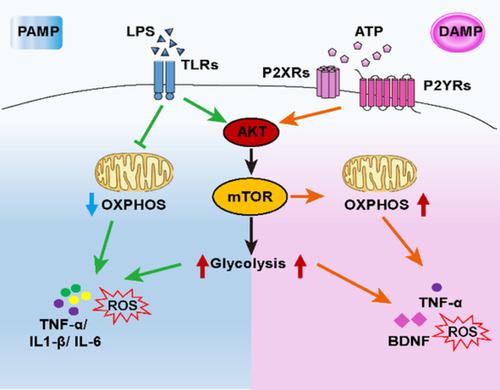
Microglia constantly survey the brain microenvironment and rapidly adopt different phenotypes in response to environmental stimuli. Such dynamic functions require a unique metabolism and bioenergetics. However, little is known about the basic metabolism of microglia and how metabolic changes regulate microglia function. Here, we uncover that microglia activation is accompanied by extensive transcriptional changes in glucose and lipid metabolism-related genes. Using metabolic flux assays, we found that LPS, a prototype of the pathogen-associated molecular patterns (PAMPs), significantly enhanced glycolysis but suppressed oxidative phosphorylation (OXPHOS) in primary cultured microglia. By contrast, ATP, a known damage-associated molecular pattern (DAMPs) that triggers sterile activation of microglia, boosted both glycolysis and OXPHOS. Importantly, both LPS and ATP activated the mechanistic target of rapamycin (mTOR) pathway and enhanced the intracellular reactive oxygen species (ROS). Inhibition of mTOR activity suppressed glycolysis and ROS production in both conditions but exerted different effects on OXPHOS: it attenuated the ATP-induced elevation of OXPHOS, yet had no impact on the LPS-induced suppression of OXPHOS. Further, inhibition of mTOR or glycolysis decreased production of LPS-induced proinflammatory cytokines and ATP-induced tumor necrosis factor-α (TNF-α) and brain derived neurotrophic factor (BDNF) in microglia. Our study reveals a critical role for mTOR in the regulation of metabolic programming of microglia to shape their distinct functions under different states and shed light on the potential application of targeting metabolism to interfere with microglia-mediated neuroinflammation in multiple disorders.
中文翻译:

mTOR介导的代谢重编程可响应脂多糖和ATP,形成不同的小胶质细胞功能。
小胶质细胞不断调查大脑的微环境,并根据环境刺激迅速采用不同的表型。这种动态功能需要独特的新陈代谢和生物能。然而,关于小胶质细胞的基本代谢以及代谢变化如何调节小胶质细胞功能的知识鲜为人知。在这里,我们发现小胶质细胞活化伴随着葡萄糖和脂质代谢相关基因的大量转录变化。使用代谢通量测定,我们发现LPS(病原体相关分子模式(PAMPs)的原型)显着增强了糖原分解,但抑制了原代培养的小胶质细胞中的氧化磷酸化(OXPHOS)。相比之下,ATP是引发小胶质细胞无菌激活的一种已知的损伤相关分子模式(DAMP),可促进糖酵解和OXPHOS。重要的,LPS和ATP均可激活雷帕霉素(mTOR)通路的机械靶标并增强细胞内活性氧(ROS)。在两种情况下,mTOR活性的抑制均抑制了糖酵解和ROS的产生,但对OXPHOS产生了不同的影响:它减弱了ATP诱导的OXPHOS的升高,但对LPS诱导的OXPHOS的抑制没有影响。此外,抑制mTOR或糖酵解降低了小胶质细胞中LPS诱导的促炎细胞因子和ATP诱导的肿瘤坏死因子-α(TNF-α)和脑源性神经营养因子(BDNF)的产生。
更新日期:2019-12-03
中文翻译:

mTOR介导的代谢重编程可响应脂多糖和ATP,形成不同的小胶质细胞功能。
小胶质细胞不断调查大脑的微环境,并根据环境刺激迅速采用不同的表型。这种动态功能需要独特的新陈代谢和生物能。然而,关于小胶质细胞的基本代谢以及代谢变化如何调节小胶质细胞功能的知识鲜为人知。在这里,我们发现小胶质细胞活化伴随着葡萄糖和脂质代谢相关基因的大量转录变化。使用代谢通量测定,我们发现LPS(病原体相关分子模式(PAMPs)的原型)显着增强了糖原分解,但抑制了原代培养的小胶质细胞中的氧化磷酸化(OXPHOS)。相比之下,ATP是引发小胶质细胞无菌激活的一种已知的损伤相关分子模式(DAMP),可促进糖酵解和OXPHOS。重要的,LPS和ATP均可激活雷帕霉素(mTOR)通路的机械靶标并增强细胞内活性氧(ROS)。在两种情况下,mTOR活性的抑制均抑制了糖酵解和ROS的产生,但对OXPHOS产生了不同的影响:它减弱了ATP诱导的OXPHOS的升高,但对LPS诱导的OXPHOS的抑制没有影响。此外,抑制mTOR或糖酵解降低了小胶质细胞中LPS诱导的促炎细胞因子和ATP诱导的肿瘤坏死因子-α(TNF-α)和脑源性神经营养因子(BDNF)的产生。











































 京公网安备 11010802027423号
京公网安备 11010802027423号