Nature Catalysis ( IF 42.8 ) Pub Date : 2019-12-02 , DOI: 10.1038/s41929-019-0387-3 Santanu Singha , Eloisa Serrano , Shobhan Mondal , Constantin G. Daniliuc , Frank Glorius
|
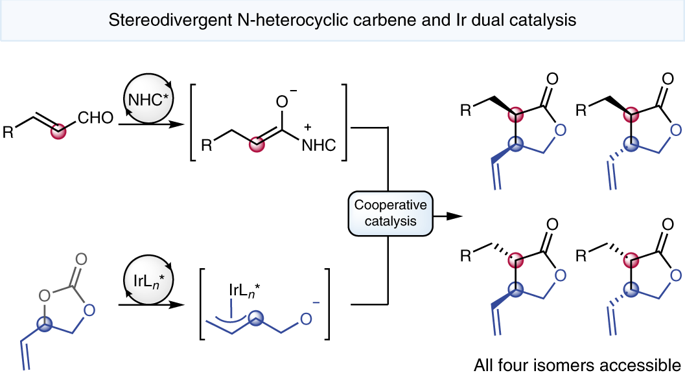
The stereodivergent synthesis of natural product frameworks via a single transformation using simple starting materials is a significant challenge. The prevalence of γ-butyrolactones in biologically active natural products has long motivated the development of enantioselective strategies towards their synthesis. Herein, we report an enantio- and diastereodivergent [3 + 2] annulation reaction for the synthesis of α,β-disubstituted γ-butyrolactones through cooperative N-heterocyclic carbene organocatalysis and iridium catalysis. This method overcomes the challenges of merging N-heterocyclic carbene organocatalysis with iridium catalysis by the appropriate choice of ligands. The use of two chiral catalysts allowed control over the relative and absolute configuration of the two formed stereocentres, thereby providing selective access to all four possible stereoisomers of the γ-lactone products. The transformation could be extended to the synthesis of δ-lactams via [4 + 2] annulation. The synthetic utility of this methodology was illustrated in the concise synthesis of the naturally occurring lignan (−)-hinokinin.
中文翻译:

N-杂环卡宾与Ir催化合成对映异构富集的α,β-二取代的γ-丁内酯
通过使用简单的起始原料的一次转化,天然产物框架的立体发散性合成是一个巨大的挑战。长期以来,γ-丁内酯在具有生物活性的天然产物中的存在一直在推动其合成对映选择性策略的发展。本文中,我们报道了通过对合N-杂环卡宾有机催化和铱催化合成α,β-二取代γ-丁内酯的对映体和非对映体[3 + 2]环化反应。此方法克服了配体的合适的选择合并用铱催化N-杂环卡宾有机催化的挑战。使用两种手性催化剂可以控制两个形成的立体中心的相对和绝对构型,从而提供对γ-内酯产物的所有四种可能的立体异构体的选择性途径。该转化可以扩展为通过[4 + 2]环化法合成δ-内酰胺。天然存在的木脂素(-)-hinokinin的简明合成中说明了该方法的合成效用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号