当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The effect of the electronic structure and flexibility of the counteranions on magnetization relaxation in [Dy(L)2(H2O)5]3+ (L = phosphine oxide derivative) pentagonal bipyramidal SIMs
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2019/12/02 , DOI: 10.1039/c9qi01412h Ismael F. Díaz-Ortega 1, 2, 3, 4, 5 , Juan Manuel Herrera 1, 2, 3, 4, 5 , Sourav Dey 6, 7, 8, 9 , Hiroyuki Nojiri 10, 11, 12, 13 , Gopalan Rajaraman 6, 7, 8, 9 , Enrique Colacio 1, 2, 3, 4, 5
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2019/12/02 , DOI: 10.1039/c9qi01412h Ismael F. Díaz-Ortega 1, 2, 3, 4, 5 , Juan Manuel Herrera 1, 2, 3, 4, 5 , Sourav Dey 6, 7, 8, 9 , Hiroyuki Nojiri 10, 11, 12, 13 , Gopalan Rajaraman 6, 7, 8, 9 , Enrique Colacio 1, 2, 3, 4, 5
Affiliation
|
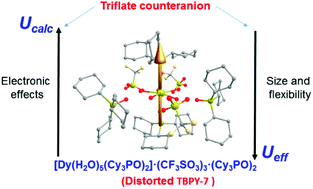
We report here a new DyIII-SIM [Dy(OPCy3)2(H2O)5](CF3SO3)3·2OPCy3 (OPCy3 = tricyclohexylphosphine oxide) with a pentagonal bipyramidal geometry, which exhibits a blocking temperature TB = 8.5 K and an anisotropy barrier Ueff = 562 K. Ab initio calculations show that this complex exhibits the largest Ucalc = 732 K among the DyIII-SIM complexes containing the [Dy(L)2(H2O)5]3+ (L = phosphine oxide derivative) cationic unit, which is essentially due to the electronic effects of the triflate anion that increase the charge difference between the oxygen atoms of the ligands L coordinated in axial positions and those belonging to the equatorial water molecules. This charge difference enhancement, which is also reflected in a larger difference between the corresponding Dy–O distances (Δ), appears to be the driving force to increase the Ucalc value. The comparatively smaller experimental Ueff value observed for this compound has been justified by the flexibility of the structural network due to the size of the triflate counteranions. The absence of a clear correlation between TB and Ueff (or Ucalc) suggests the involvement of Raman and QTM mechanisms in the magnetization relaxation process.
中文翻译:

电子结构和抗衡阴离子的挠性对[Dy(L)2(H2O)5] 3+(L =氧化膦衍生物)五边形双锥体SIM中磁化弛豫的影响
我们在这里报告一个具有五角双锥体几何形状的新Dy III -SIM [Dy(OPCy 3)2(H 2 O)5 ](CF 3 SO 3)3 ·2OPCy 3(OPCy 3 =三环己基氧化膦)温度T B = 8.5 K,各向异性势垒U eff = 562K。从头算计算表明,该配合物在包含[Dy(L)2(H 2)的Dy III -SIM配合物中表现出最大的U calc = 732K。O)5 ] 3+(L =氧化膦衍生物)阳离子单元,这主要归因于三氟甲磺酸根阴离子的电子效应,该效应增加了在轴向位置配位的配体L的氧原子与属于该位置的那些氧原子之间的电荷差赤道水分子。这种电荷差异的增强,也反映在相应的Dy-O距离(Δ)之间的较大差异中,似乎是增加U calc值的驱动力。该相对较小的实验ù EFF由于三氟甲磺酸盐抗衡阴离子的大小,结构网络的灵活性证明了对该化合物观察到的值是合理的。T B和U eff(或U calc)之间没有明确的相关性,这表明拉曼和QTM机制参与了磁化弛豫过程。
更新日期:2020-02-13
中文翻译:

电子结构和抗衡阴离子的挠性对[Dy(L)2(H2O)5] 3+(L =氧化膦衍生物)五边形双锥体SIM中磁化弛豫的影响
我们在这里报告一个具有五角双锥体几何形状的新Dy III -SIM [Dy(OPCy 3)2(H 2 O)5 ](CF 3 SO 3)3 ·2OPCy 3(OPCy 3 =三环己基氧化膦)温度T B = 8.5 K,各向异性势垒U eff = 562K。从头算计算表明,该配合物在包含[Dy(L)2(H 2)的Dy III -SIM配合物中表现出最大的U calc = 732K。O)5 ] 3+(L =氧化膦衍生物)阳离子单元,这主要归因于三氟甲磺酸根阴离子的电子效应,该效应增加了在轴向位置配位的配体L的氧原子与属于该位置的那些氧原子之间的电荷差赤道水分子。这种电荷差异的增强,也反映在相应的Dy-O距离(Δ)之间的较大差异中,似乎是增加U calc值的驱动力。该相对较小的实验ù EFF由于三氟甲磺酸盐抗衡阴离子的大小,结构网络的灵活性证明了对该化合物观察到的值是合理的。T B和U eff(或U calc)之间没有明确的相关性,这表明拉曼和QTM机制参与了磁化弛豫过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号