当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pharmacokinetics-Driven Optimization of 7-Methylimidazo[1,5-a]pyrazin-8(7H)-one as Novel BRD4 Inhibitors.
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2019-11-26 , DOI: 10.1021/acsmedchemlett.9b00474 Yifei Yang 1, 2 , Pan Chen 1 , Leilei Zhao 3 , Fangqing Zhang 3 , Huibin Zhang 3 , Jinpei Zhou 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2019-11-26 , DOI: 10.1021/acsmedchemlett.9b00474 Yifei Yang 1, 2 , Pan Chen 1 , Leilei Zhao 3 , Fangqing Zhang 3 , Huibin Zhang 3 , Jinpei Zhou 1
Affiliation
|
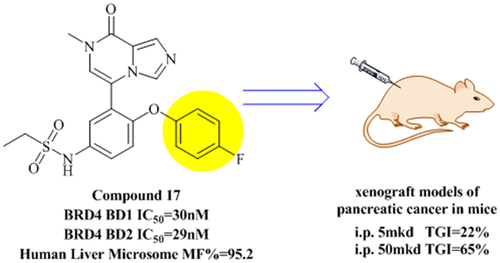
The BET bromodomain containing protein (BRD4) plays a key role in transcription regulation. Therefore, efforts to generate BRD4 inhibitors with excellent potency and DMPK properties are of clinical value. As a continuing work to improve the stability in in vitro metabolic experiments of liver microsomes of our previously reported 7-methylimidazo[1,5-a]pyrazin-8(7H)-one, our optimization of this poor pharmacokinetics focusing on the phenyl substituent is performed. Fortunately, compound 17 displayed subnanomolar potency (IC50 = 30 nM) against BRD4(1), and its liver microsome stability in human, rat, and mouse are more favorable than previously reported inhibitor 28. Compound 17 exhibited antitumor efficacy with no significant toxicity in xenograft models of pancreatic cancer. In addition, fluorescent probe and nuclei-specific dye were utilized to verify apoptosis-inducing of compound 17 via intranuclear potency in BXPC-3 cell line.
中文翻译:

作为新型BRD4抑制剂的7-甲基咪唑并[1,5-a]吡嗪-8(7H)-one的药代动力学驱动优化。
含BET溴结构域的蛋白质(BRD4)在转录调控中起关键作用。因此,努力产生具有优异效力和DMPK特性的BRD4抑制剂具有临床价值。为了改善我们先前报道的7-甲基咪唑并[1,5-a]吡嗪-8(7H)-one的肝微粒体的体外代谢实验的稳定性,我们对这种不良药代动力学的优化主要集中在苯基取代基上。被执行。幸运的是,化合物17对BRD4(1)表现出亚纳摩尔效价(IC50 = 30 nM),并且其在人,大鼠和小鼠中的肝微粒体稳定性比以前报道的抑制剂28更有利。化合物17在抗肿瘤药中没有明显的毒性。胰腺癌的异种移植模型。此外,
更新日期:2019-12-02
中文翻译:

作为新型BRD4抑制剂的7-甲基咪唑并[1,5-a]吡嗪-8(7H)-one的药代动力学驱动优化。
含BET溴结构域的蛋白质(BRD4)在转录调控中起关键作用。因此,努力产生具有优异效力和DMPK特性的BRD4抑制剂具有临床价值。为了改善我们先前报道的7-甲基咪唑并[1,5-a]吡嗪-8(7H)-one的肝微粒体的体外代谢实验的稳定性,我们对这种不良药代动力学的优化主要集中在苯基取代基上。被执行。幸运的是,化合物17对BRD4(1)表现出亚纳摩尔效价(IC50 = 30 nM),并且其在人,大鼠和小鼠中的肝微粒体稳定性比以前报道的抑制剂28更有利。化合物17在抗肿瘤药中没有明显的毒性。胰腺癌的异种移植模型。此外,











































 京公网安备 11010802027423号
京公网安备 11010802027423号