当前位置:
X-MOL 学术
›
Int. J. Mass Spectrom.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ion mobility spectrometry and theoretical study for investigation of thermal decomposition, chemical ionization, and dimer formation of proline
International Journal of Mass Spectrometry ( IF 1.6 ) Pub Date : 2020-02-01 , DOI: 10.1016/j.ijms.2019.116272 Manijeh Tozihi , Hamed Bahrami , Bahman Farajmand , Mahmoud Tabrizchi
International Journal of Mass Spectrometry ( IF 1.6 ) Pub Date : 2020-02-01 , DOI: 10.1016/j.ijms.2019.116272 Manijeh Tozihi , Hamed Bahrami , Bahman Farajmand , Mahmoud Tabrizchi
|
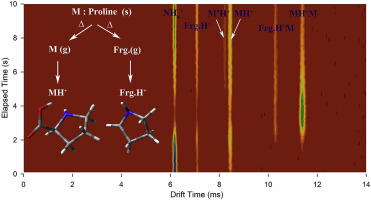
Abstract Chemical ionization and thermal decomposition of proline have been investigated by corona discharge ion mobility spectrometry (CD-IMS) and high-level ab initio calculations. Five main peaks were observed in the ion mobility spectrum of proline at low cell temperatures while only two remained at high temperatures. Experimental and theoretical evidences were collected to link the observed peaks to the related ionic species. Two peaks were assigned to the protonated proline and its symmetric proton bound dimer. Using a new mass-mobility correlation equation based on two standard mases, the other peaks were assigned to the pyrrolidine ring fragment ion with 70 amu, its asymmetric proton bound dimer with neutral proline, and a less stable protonated isomer of proline. The relative intensities of the peaks related to the fragment, parent, and the dimer ions were also explained based on the abundance of the ions in the IMS ionization region. In addition, the structure of the protonated proline was studied by considering ten stable proline conformers using ab initio calculations. Topical proton affinity values were then obtained for all the conformers. The results of the theoretical calculations showed that the proton transfer from ammonium reactant ion occurs preferably at nitrogen site of proline. The unimolecular fragmentation of protonated proline (116 amu) by eliminating H2O + CO (46 units) was also investigated by analyzing the ion mobility spectra backed by ab initio calculations. The results indicated that this reaction cannot happen in the ion mobility cell because of the high energy barrier in the reaction path. Hence, the fragment pyrrolidine ring ion is produced via thermal decomposition of proline in the injection port, followed by protonation in the ionization region.
中文翻译:

用于研究脯氨酸的热分解、化学电离和二聚体形成的离子迁移谱和理论研究
摘要 脯氨酸的化学电离和热分解已通过电晕放电离子迁移谱 (CD-IMS) 和高级从头计算进行了研究。在低细胞温度下脯氨酸的离子迁移谱中观察到五个主峰,而在高温下仅保留两个峰。收集了实验和理论证据以将观察到的峰与相关离子种类联系起来。两个峰指定给质子化脯氨酸及其对称质子结合二聚体。使用基于两个标准质量的新质量-迁移率相关方程,其他峰被指定为具有 70 amu 的吡咯烷环碎片离子、其与中性脯氨酸的不对称质子结合二聚体和不太稳定的脯氨酸质子化异构体。与片段、母体、并且还基于 IMS 电离区域中离子的丰度来解释二聚体离子。此外,通过使用 ab initio 计算考虑十个稳定的脯氨酸构象异构体,研究了质子化脯氨酸的结构。然后获得所有构象异构体的局部质子亲和力值。理论计算结果表明,铵反应物离子的质子转移优选发生在脯氨酸的氮位点。通过分析由 ab initio 计算支持的离子迁移谱,还研究了通过消除 H2O + CO(46 个单位)对质子化脯氨酸(116 amu)的单分子裂解。结果表明,由于反应路径中的高能垒,该反应不会在离子迁移池中发生。因此,
更新日期:2020-02-01
中文翻译:

用于研究脯氨酸的热分解、化学电离和二聚体形成的离子迁移谱和理论研究
摘要 脯氨酸的化学电离和热分解已通过电晕放电离子迁移谱 (CD-IMS) 和高级从头计算进行了研究。在低细胞温度下脯氨酸的离子迁移谱中观察到五个主峰,而在高温下仅保留两个峰。收集了实验和理论证据以将观察到的峰与相关离子种类联系起来。两个峰指定给质子化脯氨酸及其对称质子结合二聚体。使用基于两个标准质量的新质量-迁移率相关方程,其他峰被指定为具有 70 amu 的吡咯烷环碎片离子、其与中性脯氨酸的不对称质子结合二聚体和不太稳定的脯氨酸质子化异构体。与片段、母体、并且还基于 IMS 电离区域中离子的丰度来解释二聚体离子。此外,通过使用 ab initio 计算考虑十个稳定的脯氨酸构象异构体,研究了质子化脯氨酸的结构。然后获得所有构象异构体的局部质子亲和力值。理论计算结果表明,铵反应物离子的质子转移优选发生在脯氨酸的氮位点。通过分析由 ab initio 计算支持的离子迁移谱,还研究了通过消除 H2O + CO(46 个单位)对质子化脯氨酸(116 amu)的单分子裂解。结果表明,由于反应路径中的高能垒,该反应不会在离子迁移池中发生。因此,











































 京公网安备 11010802027423号
京公网安备 11010802027423号