Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Flexible and Extended Linker Domains Support Efficient Targeting of Heh2 to the Inner Nuclear Membrane.
Structure ( IF 4.4 ) Pub Date : 2019-12-02 , DOI: 10.1016/j.str.2019.11.003 Irina L Rempel 1 , Petra Popken 2 , Ali Ghavami 3 , Ankur Mishra 3 , Rizqiya A Hapsari 1 , Anouk H G Wolters 4 , Annemiek C Veldsink 1 , Marindy Klaassens 1 , Anne C Meinema 5 , Bert Poolman 6 , Ben N G Giepmans 4 , Patrick R Onck 3 , Anton Steen 1 , Liesbeth M Veenhoff 1
Structure ( IF 4.4 ) Pub Date : 2019-12-02 , DOI: 10.1016/j.str.2019.11.003 Irina L Rempel 1 , Petra Popken 2 , Ali Ghavami 3 , Ankur Mishra 3 , Rizqiya A Hapsari 1 , Anouk H G Wolters 4 , Annemiek C Veldsink 1 , Marindy Klaassens 1 , Anne C Meinema 5 , Bert Poolman 6 , Ben N G Giepmans 4 , Patrick R Onck 3 , Anton Steen 1 , Liesbeth M Veenhoff 1
Affiliation
|
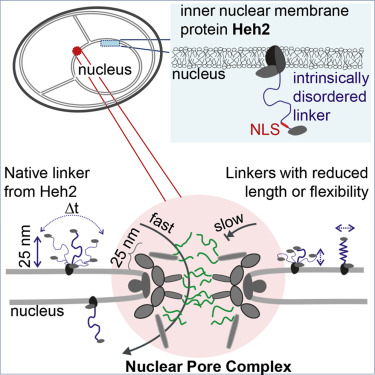
The nuclear pore complex (NPC) is embedded in the nuclear envelope and forms the main gateway to the nuclear interior including the inner nuclear membrane (INM). Two INM proteins in yeast are selectively imported. Their sorting signals consist of a nuclear localization signal, separated from the transmembrane domain by a long intrinsically disordered (ID) linker. We used computational models to predict the dynamic conformations of ID linkers and analyzed the INM targeting efficiency of proteins with linker regions with altered Stokes radii and decreased flexibilities. We find that flexibility, Stokes radius, and the frequency at which the linkers are at an extended end-to-end distance larger than 25 nm are good predictors for the targeting of the proteins. The data are consistent with a transport mechanism in which INM targeting of Heh2 is dependent on an ID linker that facilitates the crossing of the approximately 25-nm thick NPC scaffold.
中文翻译:

灵活和扩展的连接子域支持Heh2高效靶向内核膜。
核孔复合体(NPC)嵌入在核膜中,形成通往包括内部核膜(INM)在内的核内部的主要通道。酵母中的两种INM蛋白被选择性地导入。它们的分类信号由一个核定位信号组成,该信号由一个长的固有无序(ID)接头与跨膜结构域隔开。我们使用计算模型预测ID接头的动态构象,并分析了具有改变的Stokes半径和降低的柔韧性的接头区域的蛋白质的INM靶向效率。我们发现柔韧性,斯托克斯半径和接头处于延伸的端对端距离大于25 nm的频率是靶向蛋白质的良好预测指标。
更新日期:2019-12-02
中文翻译:

灵活和扩展的连接子域支持Heh2高效靶向内核膜。
核孔复合体(NPC)嵌入在核膜中,形成通往包括内部核膜(INM)在内的核内部的主要通道。酵母中的两种INM蛋白被选择性地导入。它们的分类信号由一个核定位信号组成,该信号由一个长的固有无序(ID)接头与跨膜结构域隔开。我们使用计算模型预测ID接头的动态构象,并分析了具有改变的Stokes半径和降低的柔韧性的接头区域的蛋白质的INM靶向效率。我们发现柔韧性,斯托克斯半径和接头处于延伸的端对端距离大于25 nm的频率是靶向蛋白质的良好预测指标。











































 京公网安备 11010802027423号
京公网安备 11010802027423号