Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Lipid Interactions of a Ciliary Membrane TRP Channel: Simulation and Structural Studies of Polycystin-2.
Structure ( IF 5.7 ) Pub Date : 2019-12-02 , DOI: 10.1016/j.str.2019.11.005 Qinrui Wang 1 , Robin A Corey 2 , George Hedger 2 , Prafulla Aryal 2 , Mariana Grieben 3 , Chady Nasrallah 3 , Agnese Baronina 3 , Ashley C W Pike 3 , Jiye Shi 4 , Elisabeth P Carpenter 3 , Mark S P Sansom 2
Structure ( IF 5.7 ) Pub Date : 2019-12-02 , DOI: 10.1016/j.str.2019.11.005 Qinrui Wang 1 , Robin A Corey 2 , George Hedger 2 , Prafulla Aryal 2 , Mariana Grieben 3 , Chady Nasrallah 3 , Agnese Baronina 3 , Ashley C W Pike 3 , Jiye Shi 4 , Elisabeth P Carpenter 3 , Mark S P Sansom 2
Affiliation
|
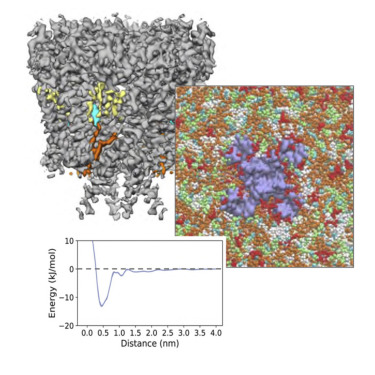
Polycystin-2 (PC2) is a transient receptor potential (TRP) channel present in ciliary membranes of the kidney. PC2 shares a transmembrane fold with other TRP channels, in addition to an extracellular domain found in TRPP and TRPML channels. Using molecular dynamics (MD) simulations and cryoelectron microscopy we identify and characterize PIP2 and cholesterol interactions with PC2. PC2 is revealed to have a PIP binding site close to the equivalent vanilloid/lipid binding site in the TRPV1 channel. A 3.0-Å structure reveals a binding site for cholesterol on PC2. Cholesterol interactions with the channel at this site are characterized by MD simulations. The two classes of lipid binding sites are compared with sites observed in other TRPs and in Kv channels. These findings suggest PC2, in common with other ion channels, may be modulated by both PIPs and cholesterol, and position PC2 within an emerging model of the roles of lipids in the regulation and organization of ciliary membranes.
中文翻译:

睫状膜 TRP 通道的脂质相互作用:多囊蛋白 2 的模拟和结构研究。
Polycystin-2 (PC2) 是一种存在于肾脏睫状膜中的瞬时受体电位 (TRP) 通道。除了在 TRPP 和 TRPML 通道中发现的细胞外结构域外,PC2 还与其他 TRP 通道共享一个跨膜折叠。使用分子动力学 (MD) 模拟和低温电子显微镜,我们识别和表征 PIP2 和胆固醇与 PC2 的相互作用。PC2 被发现有一个靠近 TRPV1 通道中等效香草/脂质结合位点的 PIP 结合位点。3.0-Å 结构揭示了 PC2 上胆固醇的结合位点。胆固醇与该位点通道的相互作用以 MD 模拟为特征。将两类脂质结合位点与在其他 TRP 和 Kv 通道中观察到的位点进行比较。这些发现表明 PC2 与其他离子通道一样,
更新日期:2019-12-02
中文翻译:

睫状膜 TRP 通道的脂质相互作用:多囊蛋白 2 的模拟和结构研究。
Polycystin-2 (PC2) 是一种存在于肾脏睫状膜中的瞬时受体电位 (TRP) 通道。除了在 TRPP 和 TRPML 通道中发现的细胞外结构域外,PC2 还与其他 TRP 通道共享一个跨膜折叠。使用分子动力学 (MD) 模拟和低温电子显微镜,我们识别和表征 PIP2 和胆固醇与 PC2 的相互作用。PC2 被发现有一个靠近 TRPV1 通道中等效香草/脂质结合位点的 PIP 结合位点。3.0-Å 结构揭示了 PC2 上胆固醇的结合位点。胆固醇与该位点通道的相互作用以 MD 模拟为特征。将两类脂质结合位点与在其他 TRP 和 Kv 通道中观察到的位点进行比较。这些发现表明 PC2 与其他离子通道一样,



























 京公网安备 11010802027423号
京公网安备 11010802027423号