当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric synthesis of 4-aryl dihydroisoquinolin-3-ones and 2-aryl morpholin-3-ones using AgOTf-activated α-bromo arylacetate
Tetrahedron ( IF 2.1 ) Pub Date : 2019-12-02 , DOI: 10.1016/j.tet.2019.130841 Su Kyung Hong , Wongi Park , Yong Sun Park
中文翻译:

AgOTf活化的α-溴代芳基乙酸酯不对称合成4-芳基二氢异喹啉-3-酮和2-芳基吗啉-3-酮
更新日期:2019-12-02
Tetrahedron ( IF 2.1 ) Pub Date : 2019-12-02 , DOI: 10.1016/j.tet.2019.130841 Su Kyung Hong , Wongi Park , Yong Sun Park
|
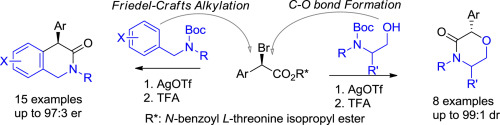
A novel asymmetric synthetic strategy to prepare 4-aryl-dihydroisoquinolinones has been developed through a highly regioselective Friedel-Crafts alkylation of benzylamine derivatives with (αR)-α-bromo arylacetates and subsequent facile lactamization. In addition, an efficient asymmetric synthesis of 2-aryl morpholinones is demonstrated with 2-aminoethanol derivatives using the same convenient substitution-lactamization protocol.
中文翻译:

AgOTf活化的α-溴代芳基乙酸酯不对称合成4-芳基二氢异喹啉-3-酮和2-芳基吗啉-3-酮
通过用(αR)-α-溴代芳基乙酸酯对苄胺衍生物进行高度区域选择性的Friedel-Crafts烷基化反应,并随后进行轻松的内酰胺化反应,已开发出制备4-芳基-二氢异喹啉酮的新型不对称合成策略。另外,使用相同的方便取代-内酰胺化方案,证明了2-氨基乙醇衍生物能有效地不对称合成2-芳基吗啉酮。











































 京公网安备 11010802027423号
京公网安备 11010802027423号