Molecular Psychiatry ( IF 9.6 ) Pub Date : 2019-12-02 , DOI: 10.1038/s41380-019-0602-2 A B Niculescu 1, 2, 3 , H Le-Niculescu 1 , K Roseberry 1 , S Wang 1, 3, 4 , J Hart 1 , A Kaur 1 , H Robertson 1 , T Jones 3 , A Strasburger 3 , A Williams 3, 5 , S M Kurian 5 , B Lamb 2 , A Shekhar 1 , D K Lahiri 1, 4 , A J Saykin 4
|
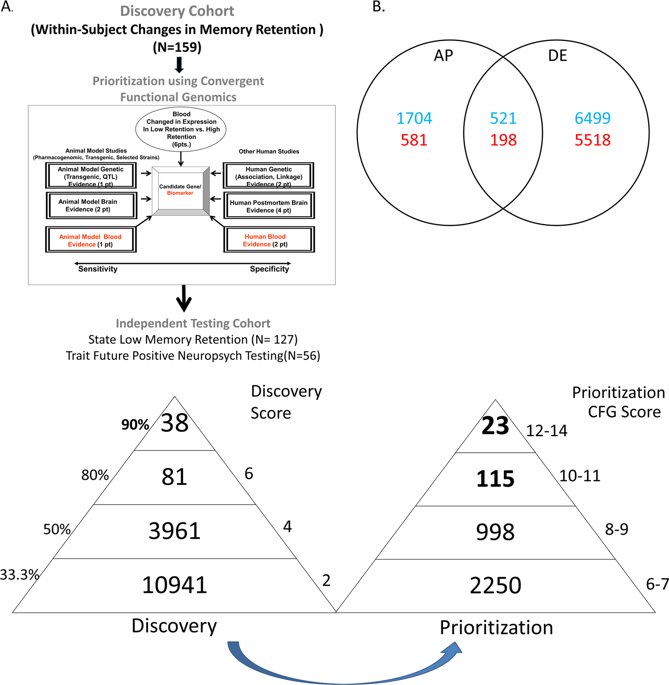
Short-term memory dysfunction is a key early feature of Alzheimer’s disease (AD). Psychiatric patients may be at higher risk for memory dysfunction and subsequent AD due to the negative effects of stress and depression on the brain. We carried out longitudinal within-subject studies in male and female psychiatric patients to discover blood gene expression biomarkers that track short term memory as measured by the retention measure in the Hopkins Verbal Learning Test. These biomarkers were subsequently prioritized with a convergent functional genomics approach using previous evidence in the field implicating them in AD. The top candidate biomarkers were then tested in an independent cohort for ability to predict state short-term memory, and trait future positive neuropsychological testing for cognitive impairment. The best overall evidence was for a series of new, as well as some previously known genes, which are now newly shown to have functional evidence in humans as blood biomarkers: RAB7A, NPC2, TGFB1, GAP43, ARSB, PER1, GUSB, and MAPT. Additional top blood biomarkers include GSK3B, PTGS2, APOE, BACE1, PSEN1, and TREM2, well known genes implicated in AD by previous brain and genetic studies, in humans and animal models, which serve as reassuring de facto positive controls for our whole-genome gene expression discovery approach. Biological pathway analyses implicate LXR/RXR activation, neuroinflammation, atherosclerosis signaling, and amyloid processing. Co-directionality of expression data provide new mechanistic insights that are consistent with a compensatory/scarring scenario for brain pathological changes. A majority of top biomarkers also have evidence for involvement in other psychiatric disorders, particularly stress, providing a molecular basis for clinical co-morbidity and for stress as an early precipitant/risk factor. Some of them are modulated by existing drugs, such as antidepressants, lithium and omega-3 fatty acids. Other drug and nutraceutical leads were identified through bioinformatic drug repurposing analyses (such as pioglitazone, levonorgestrel, salsolidine, ginkgolide A, and icariin). Our work contributes to the overall pathophysiological understanding of memory disorders and AD. It also opens new avenues for precision medicine- diagnostics (assement of risk) as well as early treatment (pharmacogenomically informed, personalized, and preventive).
中文翻译:

记忆的血液生物标志物:早期检测阿尔茨海默病、药物基因组学和重新利用药物的风险。
短期记忆功能障碍是阿尔茨海默病 (AD) 的一个关键早期特征。由于压力和抑郁对大脑的负面影响,精神病患者出现记忆功能障碍和随后的阿尔茨海默病的风险可能更高。我们对男性和女性精神病患者进行了纵向受试者内研究,以发现血液基因表达生物标志物,这些生物标志物可以跟踪短期记忆(通过霍普金斯言语学习测试中的保留测量来测量)。随后,利用该领域先前涉及 AD 的证据,通过聚合功能基因组学方法对这些生物标志物进行优先排序。然后,在一个独立的队列中对最重要的候选生物标志物进行测试,以评估其预测状态短期记忆的能力,以及未来对认知障碍进行积极的神经心理学测试的能力。最好的总体证据是一系列新基因以及一些先前已知的基因,这些基因现在新近被证明在人类中具有作为血液生物标志物的功能证据:RAB7A、NPC2、TGFB1、GAP43、ARSB、PER1、GUSB 和 MAPT 。其他顶级血液生物标志物包括 GSK3B、PTGS2、APOE、BACE1、PSEN1 和 TREM2,这些众所周知的基因与 AD 相关,之前的大脑和遗传学研究在人类和动物模型中进行了研究,它们可以作为我们全基因组事实上的阳性对照基因表达发现方法。生物通路分析涉及 LXR/RXR 激活、神经炎症、动脉粥样硬化信号传导和淀粉样蛋白加工。表达数据的同向性提供了新的机制见解,与大脑病理变化的补偿/疤痕场景一致。 大多数顶级生物标志物也有证据表明与其他精神疾病有关,特别是压力,为临床共病和压力作为早期促发/危险因素提供了分子基础。其中一些是由现有药物调节的,例如抗抑郁药、锂和 omega-3 脂肪酸。通过生物信息学药物再利用分析确定了其他药物和营养保健品的先导药物(例如吡格列酮、左炔诺孕酮、沙固体碱、银杏内酯 A 和淫羊藿苷)。我们的工作有助于对记忆障碍和 AD 的整体病理生理学理解。它还为精准医学诊断(风险评估)以及早期治疗(药物基因组学、个性化和预防)开辟了新途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号