当前位置:
X-MOL 学术
›
J. Proteomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Proteomics fingerprints of systemic mechanisms of adaptation to bile in Lactobacillus fermentum.
Journal of Proteomics ( IF 2.8 ) Pub Date : 2019-12-02 , DOI: 10.1016/j.jprot.2019.103600 Syed Azmal Ali 1 , Parul Singh 1 , Sudhir K Tomar 2 , Ashok K Mohanty 1 , Pradip Behare 2
Journal of Proteomics ( IF 2.8 ) Pub Date : 2019-12-02 , DOI: 10.1016/j.jprot.2019.103600 Syed Azmal Ali 1 , Parul Singh 1 , Sudhir K Tomar 2 , Ashok K Mohanty 1 , Pradip Behare 2
Affiliation
|
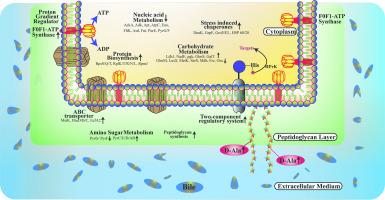
Lactobacillus fermentum is a natural resident of the human GIT and is used as a probiotic. A unique property of L. fermentum is its ability to tolerate, colonize, and survive in the harsh conditions of bile, which facilitates transient colonization of the host colon. In the current study, we investigated the key mechanisms of action involved in bacterial survival in the presence of bile, using high-resolution mass spectrometry. A total of 1071 proteins were identified, among which 378 were up-regulated and 368 down-regulated by ≥2-fold (t-test, p < .05). Differentially regulated proteins comprised both intracellular and surface-exposed (i.e., membrane) proteins (p < .01, t-test for total proteome analysis; p < .05, t-test for membrane proteome analysis). These alterations strengthen the cell envelope and also mediate bile efflux by adjusting carbohydrate metabolic pathways and prevention of protein misfolding. These processes are mainly involved in the active removal of bile salts or amelioration of its adverse effects on cells. Further investigation of mRNA transcript expression levels of selected proteins by quantitative reverse transcriptase-PCR verified the proteomic data. Together, our proteomics findings reveal the roles of post-stress recovery proteins and highlight the interacting pathways responsible for bacterial cell tolerance to bile stress. BIOLOGICAL SIGNIFICANCE: Our intestinal tract is a nutrient-rich milieu crowded with up to 100 trillion (1014) of microbes. The fact that we are born germ-free describes that these microbes must colonize our intestinal tract from outside. However, their survival is also complicated because of hazardous conditions in the gut due to the presence of bile acid and others, which exerts a deleterious effect on the beneficial microbial load. While there was limited information available describing the comprehensive mechanism of survival? Furthermore, the imbalance of these micro floras leads to numerous disease conditions. It explains the need for enhanced understanding of host-microbe interactions in the colon. The present study majorly focuses on identifying "how microbes respond to environmental stressors" in this context, particularly bile acid response. This work addresses a fascinating cellular mechanism involved in the complex changes of bile induction in the microbial system; in this case, L. fermentum NCDC 605 a well established probiotic organism. In this article, we decipher the characteristic adaptation mechanism adjusted by probiotics in the harsh condition of 1.2% bile. The generated new knowledge will also improve the potential therapeutic efficacy of probiotics strains in clinical trials for patients of inflammatory bowel diseases (IBD) and related disorders.
中文翻译:

蛋白质组学指纹图谱适应发酵乳杆菌中胆汁的系统机制。
发酵乳杆菌是人GIT的天然居民,并被用作益生菌。发酵乳杆菌的独特性质是其在苛刻的胆汁条件下耐受,定殖和生存的能力,这有利于宿主结肠的瞬时定殖。在当前的研究中,我们使用高分辨率质谱法研究了在胆汁存在下细菌存活所涉及的关键作用机制。总共鉴定出1071种蛋白质,其中378份上调≥两倍,而368份下调(t检验,p <.05)。差异调节的蛋白质包括细胞内和表面暴露(即膜)蛋白质(p <.01,用于总蛋白质组分析的t检验; p <.05,用于膜蛋白质组分析的t检验)。这些改变通过调节碳水化合物的代谢途径和防止蛋白质错误折叠来增强细胞包膜并介导胆汁外流。这些过程主要涉及胆汁盐的主动去除或减轻其对细胞的不利影响。通过定量逆转录酶-PCR进一步研究所选蛋白质的mRNA转录表达水平,验证了蛋白质组学数据。在一起,我们的蛋白质组学研究结果揭示了应激后恢复蛋白的作用,并强调了导致细菌细胞耐受胆汁应激的相互作用途径。生物学意义:我们的肠道是营养丰富的环境,挤满了多达100万亿(1014)个微生物。我们出生时是无菌的,这说明这些微生物必须从外面在我们的肠道中定植。然而,由于胆汁酸等的存在,肠道中存在危险条件,因此它们的存活也很复杂,这会对有益的微生物负荷产生有害影响。尽管可用的信息有限,无法描述生存的综合机制?此外,这些微生物区系的失衡导致许多疾病状况。它解释了需要加强对结肠中宿主-微生物相互作用的了解。在这种情况下,本研究主要集中于确定“微生物如何对环境压力源作出反应”,特别是胆汁酸反应。这项工作解决了一个令人着迷的细胞机制,涉及微生物系统中胆汁诱导的复杂变化。在这种情况下,发酵乳杆菌NCDC 605是一种成熟的益生菌。在本文中,我们破译了在1.2%胆汁的恶劣条件下益生菌调节的特征适应机制。所产生的新知识还将改善益生菌菌株在炎症性肠病(IBD)和相关疾病患者的临床试验中的潜在治疗功效。
更新日期:2019-12-02
中文翻译:

蛋白质组学指纹图谱适应发酵乳杆菌中胆汁的系统机制。
发酵乳杆菌是人GIT的天然居民,并被用作益生菌。发酵乳杆菌的独特性质是其在苛刻的胆汁条件下耐受,定殖和生存的能力,这有利于宿主结肠的瞬时定殖。在当前的研究中,我们使用高分辨率质谱法研究了在胆汁存在下细菌存活所涉及的关键作用机制。总共鉴定出1071种蛋白质,其中378份上调≥两倍,而368份下调(t检验,p <.05)。差异调节的蛋白质包括细胞内和表面暴露(即膜)蛋白质(p <.01,用于总蛋白质组分析的t检验; p <.05,用于膜蛋白质组分析的t检验)。这些改变通过调节碳水化合物的代谢途径和防止蛋白质错误折叠来增强细胞包膜并介导胆汁外流。这些过程主要涉及胆汁盐的主动去除或减轻其对细胞的不利影响。通过定量逆转录酶-PCR进一步研究所选蛋白质的mRNA转录表达水平,验证了蛋白质组学数据。在一起,我们的蛋白质组学研究结果揭示了应激后恢复蛋白的作用,并强调了导致细菌细胞耐受胆汁应激的相互作用途径。生物学意义:我们的肠道是营养丰富的环境,挤满了多达100万亿(1014)个微生物。我们出生时是无菌的,这说明这些微生物必须从外面在我们的肠道中定植。然而,由于胆汁酸等的存在,肠道中存在危险条件,因此它们的存活也很复杂,这会对有益的微生物负荷产生有害影响。尽管可用的信息有限,无法描述生存的综合机制?此外,这些微生物区系的失衡导致许多疾病状况。它解释了需要加强对结肠中宿主-微生物相互作用的了解。在这种情况下,本研究主要集中于确定“微生物如何对环境压力源作出反应”,特别是胆汁酸反应。这项工作解决了一个令人着迷的细胞机制,涉及微生物系统中胆汁诱导的复杂变化。在这种情况下,发酵乳杆菌NCDC 605是一种成熟的益生菌。在本文中,我们破译了在1.2%胆汁的恶劣条件下益生菌调节的特征适应机制。所产生的新知识还将改善益生菌菌株在炎症性肠病(IBD)和相关疾病患者的临床试验中的潜在治疗功效。











































 京公网安备 11010802027423号
京公网安备 11010802027423号