当前位置:
X-MOL 学术
›
Clin. Chim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Validation and evaluation of four sample preparation methods for the quantification of intracellular tacrolimus in peripheral blood mononuclear cells by UHPLC-MS/MS.
Clinica Chimica Acta ( IF 3.2 ) Pub Date : 2019-11-30 , DOI: 10.1016/j.cca.2019.11.033 Lisanne N van Merendonk 1 , Pere Fontova 1 , Raül Rigo-Bonnin 2 , Helena Colom 3 , Anna Vidal-Alabró 1 , Oriol Bestard 1 , Juan Torras 1 , Josep M Cruzado 1 , Josep M Grinyó 1 , Núria Lloberas 1
Clinica Chimica Acta ( IF 3.2 ) Pub Date : 2019-11-30 , DOI: 10.1016/j.cca.2019.11.033 Lisanne N van Merendonk 1 , Pere Fontova 1 , Raül Rigo-Bonnin 2 , Helena Colom 3 , Anna Vidal-Alabró 1 , Oriol Bestard 1 , Juan Torras 1 , Josep M Cruzado 1 , Josep M Grinyó 1 , Núria Lloberas 1
Affiliation
|
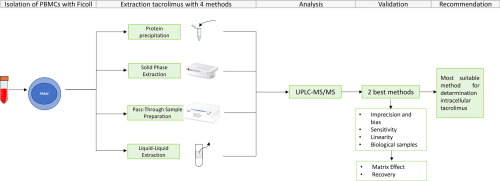
Rejection and toxicity occur despite monitoring of tacrolimus blood levels during clinical routine. The intracellular concentration in lymphocytes could be a better reflection of the tacrolimus exposure. Four extraction methods for tacrolimus in peripheral blood mononuclear cells were validated and evaluated with UHPLC-MS/MS. Methods based on protein precipitation (method 1), solid phase extraction (method 2), phospholipids and proteins removal (method 3) and liquid-liquid extraction (method 4) were evaluated on linearity, lower limit of quantification (LLOQ), imprecision and bias. Validation was completed for the methods within these requirements, adding matrix effect and recovery. Linearity was 0.126 (LLOQ)-15 µg/L, 0.504 (LLOQ)-15 µg/L and 0.298 (LLOQ)-15 µg/L with method 1, 2 and 3, respectively. With method 4 non-linearity and a LLOQ higher than 0.504 µg/L were observed. Inter-day imprecision and bias were ≤4.6%, ≤10.9%; ≤6.8%, ≤-11.2%; ≤9.4%, ≤10.3% and ≤44.6%, ≤23.1%, respectively, with methods 1, 2, 3 and 4. Validation was completed for method 1 and 3 adding matrix effect (7.6%; 15.0%) and recovery (8.9%; 10.8%), respectively. The most suitable UHPLC-MS/MS method for quantification of intracellular tacrolimus was protein precipitation due to the best performance characteristics and the least time-consuming rate and complexity.
中文翻译:

四种样品制备方法的验证和评估,用于通过UHPLC-MS / MS定量测定外周血单核细胞中的细胞内他克莫司。
尽管在临床常规期间监测了他克莫司的血药浓度,但仍发生排斥反应和毒性反应。淋巴细胞中的细胞内浓度可能是他克莫司暴露的更好反映。用UHPLC-MS / MS验证和评估了外周血单核细胞中他克莫司的四种提取方法。评价了基于蛋白质沉淀(方法1),固相萃取(方法2),磷脂和蛋白质去除(方法3)和液-液萃取(方法4)的方法的线性,定量下限(LLOQ),不精确度和偏见。已完成对这些要求内方法的验证,从而增加了基质效应和回收率。方法1、2和3的线性分别为0.126(LLOQ)-15 µg / L,0.504(LLOQ)-15 µg / L和0.298(LLOQ)-15 µg / L。用方法4观察到非线性和LLOQ高于0.504 µg / L。日间不精确度和偏倚分别为≤4.6%,≤10.9%;≤6.8%,≤-11.2%; 用方法1、2、3和4分别≤9.4%,≤10.3%和≤44.6%,≤23.1%,完成了对方法1和3的验证,增加了基质效应(7.6%; 15.0%)和回收率(8.9) %; 10.8%)。由于最佳的性能特征,最少的耗时率和复杂性,最适合用于定量细胞内他克莫司的UHPLC-MS / MS方法是蛋白质沉淀。
更新日期:2019-11-30
中文翻译:

四种样品制备方法的验证和评估,用于通过UHPLC-MS / MS定量测定外周血单核细胞中的细胞内他克莫司。
尽管在临床常规期间监测了他克莫司的血药浓度,但仍发生排斥反应和毒性反应。淋巴细胞中的细胞内浓度可能是他克莫司暴露的更好反映。用UHPLC-MS / MS验证和评估了外周血单核细胞中他克莫司的四种提取方法。评价了基于蛋白质沉淀(方法1),固相萃取(方法2),磷脂和蛋白质去除(方法3)和液-液萃取(方法4)的方法的线性,定量下限(LLOQ),不精确度和偏见。已完成对这些要求内方法的验证,从而增加了基质效应和回收率。方法1、2和3的线性分别为0.126(LLOQ)-15 µg / L,0.504(LLOQ)-15 µg / L和0.298(LLOQ)-15 µg / L。用方法4观察到非线性和LLOQ高于0.504 µg / L。日间不精确度和偏倚分别为≤4.6%,≤10.9%;≤6.8%,≤-11.2%; 用方法1、2、3和4分别≤9.4%,≤10.3%和≤44.6%,≤23.1%,完成了对方法1和3的验证,增加了基质效应(7.6%; 15.0%)和回收率(8.9) %; 10.8%)。由于最佳的性能特征,最少的耗时率和复杂性,最适合用于定量细胞内他克莫司的UHPLC-MS / MS方法是蛋白质沉淀。











































 京公网安备 11010802027423号
京公网安备 11010802027423号