当前位置:
X-MOL 学术
›
Process Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Studies on the interactions between nicosulfuron and degradation enzymes
Process Biochemistry ( IF 3.7 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.procbio.2019.11.038 Zhe Zhang , Dongchen Yang , Jiaying Wang , Jingqian Huo , Jinlin Zhang
Process Biochemistry ( IF 3.7 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.procbio.2019.11.038 Zhe Zhang , Dongchen Yang , Jiaying Wang , Jingqian Huo , Jinlin Zhang
|
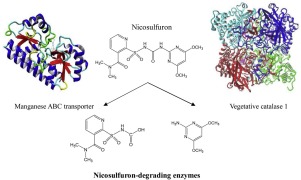
Abstract The interactions between nicosulfuron and two degradation enzymes (vegetative catalase 1 and manganese ABC transporter) from the Bacillus subtilis YB1 strain were studied and molecular docking simulations and surface plasmon resonance (SPR) were used to research their specific interaction patterns and affinities. The results showed that vegetative catalase 1 and manganese ABC transporter bound specifically to nicosulfuron and that the former binding ability was stronger than that of the latter. The manganese ABC transporter mainly interacted with nicosulfuron by strong hydrophobic interactions and hydrogen bonds (oxyanion hole), while vegetative catalase 1 formed a strong hydrophobic interaction with nicosulfuron in its main channel and hydrogen bond with nicosulfuron in the side chains. Vegetative catalase 1 and manganese ABC transporter catalyze nicosulfuron degradation, and molecular docking simulations and SPR are good methods for studying molecular interactions, which could make a foundation for the study of degradation mechanisms of enzymes.
中文翻译:

烟嘧磺隆与降解酶相互作用的研究
摘要 研究了烟嘧磺隆与枯草芽孢杆菌 YB1 菌株的两种降解酶(植物性过氧化氢酶 1 和锰 ABC 转运蛋白)之间的相互作用,并利用分子对接模拟和表面等离子体共振 (SPR) 研究了它们的特异性相互作用模式和亲和力。结果表明,营养过氧化氢酶1和锰ABC转运蛋白与烟嘧磺隆特异性结合,且前者的结合能力强于后者。锰ABC转运蛋白主要通过强疏水相互作用和氢键(氧阴离子孔)与烟嘧磺隆相互作用,而营养过氧化氢酶1在其主要通道与烟嘧磺隆形成强疏水相互作用,在侧链与烟嘧磺隆形成氢键。
更新日期:2020-04-01
中文翻译:

烟嘧磺隆与降解酶相互作用的研究
摘要 研究了烟嘧磺隆与枯草芽孢杆菌 YB1 菌株的两种降解酶(植物性过氧化氢酶 1 和锰 ABC 转运蛋白)之间的相互作用,并利用分子对接模拟和表面等离子体共振 (SPR) 研究了它们的特异性相互作用模式和亲和力。结果表明,营养过氧化氢酶1和锰ABC转运蛋白与烟嘧磺隆特异性结合,且前者的结合能力强于后者。锰ABC转运蛋白主要通过强疏水相互作用和氢键(氧阴离子孔)与烟嘧磺隆相互作用,而营养过氧化氢酶1在其主要通道与烟嘧磺隆形成强疏水相互作用,在侧链与烟嘧磺隆形成氢键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号