当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A scaffold replacement approach towards new sirtuin 2 inhibitors.
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2019-11-30 , DOI: 10.1016/j.bmc.2019.115231 Tina Seifert 1 , Marcus Malo 1 , Tarja Kokkola 2 , E Johanna L Stéen 1 , Kristian Meinander 3 , Erik A A Wallén 3 , Elina M Jarho 2 , Kristina Luthman 1
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2019-11-30 , DOI: 10.1016/j.bmc.2019.115231 Tina Seifert 1 , Marcus Malo 1 , Tarja Kokkola 2 , E Johanna L Stéen 1 , Kristian Meinander 3 , Erik A A Wallén 3 , Elina M Jarho 2 , Kristina Luthman 1
Affiliation
|
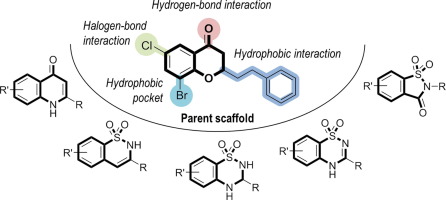
Sirtuins (SIRT1-SIRT7) are an evolutionary conserved family of NAD+-dependent protein deacylases regulating the acylation state of ε-N-lysine residues of proteins thereby controlling key biological processes. Numerous studies have found association of the aberrant enzymatic activity of SIRTs with various diseases like diabetes, cancer and neurodegenerative disorders. Previously, we have shown that substituted 2-alkyl-chroman-4-one/chromone derivatives can serve as selective inhibitors of SIRT2 possessing an antiproliferative effect in two human cancer cell lines. In this study, we have explored the bioisosteric replacement of the chroman-4-one/chromone core structure with different less lipophilic bicyclic scaffolds to overcome problems associated to poor physiochemical properties due to a highly lipophilic substitution pattern required for achieve a good inhibitory effect. Various new derivatives based on the quinolin-4(1H)-one scaffold, bicyclic secondary sulfonamides or saccharins were synthesized and evaluated for their SIRT inhibitory effect. Among the evaluated scaffolds, the benzothiadiazine-1,1-dioxide-based compounds showed the highest SIRT2 inhibitory activity. Molecular modeling studies gave insight into the binding mode of the new scaffold-replacement analogues.
中文翻译:

新型sirtuin 2抑制剂的支架替代方法。
Sirtuins(SIRT1-SIRT7)是一个进化保守的NAD +依赖性蛋白去酰基化家族,可调节蛋白ε-N-赖氨酸残基的酰化状态,从而控制关键的生物学过程。大量研究发现,SIRTs的异常酶促活性与各种疾病(例如糖尿病,癌症和神经退行性疾病)相关。以前,我们已经表明,取代的2-烷基-苯并吡喃-4-酮/苯并二氢吡喃酮衍生物可以作为SIRT2的选择性抑制剂,在两种人类癌细胞系中具有抗增殖作用。在这项研究中,我们已经探索了用不同的较少亲脂性双环支架替代chroman-4-one / chromone核心结构的生物立体替代,以克服由于获得良好抑制效果所需的高度亲脂性取代模式而导致的理化性质差的问题。合成了基于喹啉4(1H)-一支架,双环仲磺酰胺或糖精的各种新衍生物,并评估了其对SIRT的抑制作用。在评估的支架中,基于苯并噻二嗪-1,1-二氧化物的化合物显示出最高的SIRT2抑制活性。分子建模研究提供了新的支架替代类似物的绑定模式的见解。合成了双环仲磺酰胺或糖精,并评估了其对SIRT的抑制作用。在评估的支架中,基于苯并噻二嗪-1,1-二氧化物的化合物显示出最高的SIRT2抑制活性。分子建模研究提供了新的支架替代类似物的绑定模式的见解。合成了双环仲磺酰胺或糖精,并评估了其对SIRT的抑制作用。在评估的支架中,基于苯并噻二嗪-1,1-二氧化物的化合物显示出最高的SIRT2抑制活性。分子建模研究提供了新的支架替代类似物的绑定模式的见解。
更新日期:2019-11-30
中文翻译:

新型sirtuin 2抑制剂的支架替代方法。
Sirtuins(SIRT1-SIRT7)是一个进化保守的NAD +依赖性蛋白去酰基化家族,可调节蛋白ε-N-赖氨酸残基的酰化状态,从而控制关键的生物学过程。大量研究发现,SIRTs的异常酶促活性与各种疾病(例如糖尿病,癌症和神经退行性疾病)相关。以前,我们已经表明,取代的2-烷基-苯并吡喃-4-酮/苯并二氢吡喃酮衍生物可以作为SIRT2的选择性抑制剂,在两种人类癌细胞系中具有抗增殖作用。在这项研究中,我们已经探索了用不同的较少亲脂性双环支架替代chroman-4-one / chromone核心结构的生物立体替代,以克服由于获得良好抑制效果所需的高度亲脂性取代模式而导致的理化性质差的问题。合成了基于喹啉4(1H)-一支架,双环仲磺酰胺或糖精的各种新衍生物,并评估了其对SIRT的抑制作用。在评估的支架中,基于苯并噻二嗪-1,1-二氧化物的化合物显示出最高的SIRT2抑制活性。分子建模研究提供了新的支架替代类似物的绑定模式的见解。合成了双环仲磺酰胺或糖精,并评估了其对SIRT的抑制作用。在评估的支架中,基于苯并噻二嗪-1,1-二氧化物的化合物显示出最高的SIRT2抑制活性。分子建模研究提供了新的支架替代类似物的绑定模式的见解。合成了双环仲磺酰胺或糖精,并评估了其对SIRT的抑制作用。在评估的支架中,基于苯并噻二嗪-1,1-二氧化物的化合物显示出最高的SIRT2抑制活性。分子建模研究提供了新的支架替代类似物的绑定模式的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号