Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Weekly dose-dense chemotherapy in first-line epithelial ovarian, fallopian tube, or primary peritoneal carcinoma treatment (ICON8): primary progression free survival analysis results from a GCIG phase 3 randomised controlled trial.
The Lancet ( IF 98.4 ) Pub Date : 2019-11-29 , DOI: 10.1016/s0140-6736(19)32259-7 Andrew R Clamp 1 , Elizabeth C James 2 , Iain A McNeish 3 , Andrew Dean 4 , Jae-Weon Kim 5 , Dearbhaile M O'Donnell 6 , Jane Hook 7 , Christopher Coyle 8 , Sarah Blagden 9 , James D Brenton 10 , Raj Naik 11 , Tim Perren 7 , Sudha Sundar 12 , Adrian D Cook 2 , Gosala S Gopalakrishnan 2 , Hani Gabra 13 , Rosemary Lord 14 , Graham Dark 15 , Helena M Earl 16 , Marcia Hall 17 , Susana Banerjee 18 , Rosalind M Glasspool 19 , Rachel Jones 20 , Sarah Williams 21 , Ann Marie Swart 22 , Sally Stenning 2 , Mahesh Parmar 2 , Richard Kaplan 2 , Jonathan A Ledermann 23
The Lancet ( IF 98.4 ) Pub Date : 2019-11-29 , DOI: 10.1016/s0140-6736(19)32259-7 Andrew R Clamp 1 , Elizabeth C James 2 , Iain A McNeish 3 , Andrew Dean 4 , Jae-Weon Kim 5 , Dearbhaile M O'Donnell 6 , Jane Hook 7 , Christopher Coyle 8 , Sarah Blagden 9 , James D Brenton 10 , Raj Naik 11 , Tim Perren 7 , Sudha Sundar 12 , Adrian D Cook 2 , Gosala S Gopalakrishnan 2 , Hani Gabra 13 , Rosemary Lord 14 , Graham Dark 15 , Helena M Earl 16 , Marcia Hall 17 , Susana Banerjee 18 , Rosalind M Glasspool 19 , Rachel Jones 20 , Sarah Williams 21 , Ann Marie Swart 22 , Sally Stenning 2 , Mahesh Parmar 2 , Richard Kaplan 2 , Jonathan A Ledermann 23
Affiliation
|
BACKGROUND
Carboplatin and paclitaxel administered every 3 weeks is standard-of-care first-line chemotherapy for epithelial ovarian cancer. The Japanese JGOG3016 trial showed a significant improvement in progression-free and overall survival with dose-dense weekly paclitaxel and 3-weekly carboplatin. In this study, we aimed to compare efficacy and safety of two dose-dense weekly regimens to standard 3-weekly chemotherapy in a predominantly European population with epithelial ovarian cancer.
METHODS
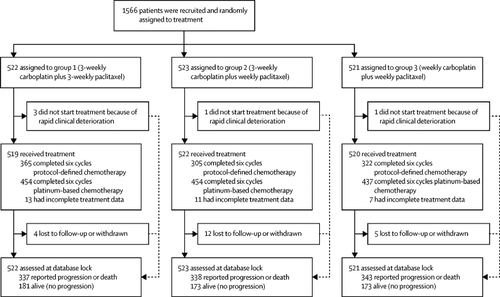
In this phase 3 trial, women with newly diagnosed International Federation of Gynecology and Obstetrics stage IC-IV epithelial ovarian cancer were randomly assigned to group 1 (carboplatin area under the curve [AUC]5 or AUC6 and 175 mg/m2 paclitaxel every 3 weeks), group 2 (carboplatin AUC5 or AUC6 every 3 weeks and 80 mg/m2 paclitaxel weekly), or group 3 (carboplatin AUC2 and 80 mg/m2 paclitaxel weekly). Written informed consent was provided by all women who entered the trial. The protocol had the appropriate national research ethics committee approval for the countries where the study was conducted. Patients entered the trial after immediate primary surgery, or before neoadjuvant chemotherapy with subsequent planned delayed primary surgery. The trial coprimary outcomes were progression-free survival and overall survival. Data analyses were done on an intention-to-treat basis, and were powered to detect a hazard ratio of 0·75 in progression-free survival. The main comparisons were between the control group (group 1) and each of the weekly research groups (groups 2 and 3).
FINDINGS
Between June 6, 2011, and Nov 28, 2014, 1566 women were randomly assigned to treatment. 72% (365), completed six protocol-defined treatment cycles in group 1, 60% (305) in group 2, and 63% (322) in group 3, although 90% (454), 89% (454), and 85% (437) completed six platinum-based chemotherapy cycles, respectively. Paclitaxel dose intensification was achieved with weekly treatment (median total paclitaxel dose 1010 mg/m2 in group 1; 1233 mg/m2 in group 2; 1274 mg/m2 in group 3). By February, 2017, 1018 (65%) patients had experienced disease progression. No significant progression-free survival increase was observed with either weekly regimen (restricted mean survival time 24·4 months [97·5% CI 23·0-26·0] in group 1, 24·9 months [24·0-25·9] in group 2, 25·3 months [23·9-26·9] in group 3; median progression-free survival 17·7 months [IQR 10·6-not reached] in group 1, 20·8 months [11·9-59·0] in group 2, 21·0 months [12·0-54·0] in group 3; log-rank p=0·35 for group 2 vs group 1; group 3 vs 1 p=0·51). Although grade 3 or 4 toxic effects increased with weekly treatment, these effects were predominantly uncomplicated. Febrile neutropenia and sensory neuropathy incidences were similar across groups.
INTERPRETATION
Weekly dose-dense chemotherapy can be delivered successfully as first-line treatment for epithelial ovarian cancer but does not significantly improve progression-free survival compared with standard 3-weekly chemotherapy in predominantly European populations.
FUNDING
Cancer Research UK, Medical Research Council, Health Research Board in Ireland, Irish Cancer Society, Cancer Australia.
中文翻译:

一线上皮性卵巢癌、输卵管癌或原发性腹膜癌治疗中的每周剂量密集化疗 (ICON8):来自 GCIG 3 期随机对照试验的原发性无进展生存分析结果。
背景 每 3 周施用卡铂和紫杉醇是上皮性卵巢癌的标准一线化疗。日本 JGOG3016 试验显示,剂量密集的每周紫杉醇和每 3 周卡铂可显着改善无进展生存期和总生存期。在这项研究中,我们的目的是在主要患有上皮性卵巢癌的欧洲人群中比较两种剂量密集每周化疗方案与标准每周三周化疗的疗效和安全性。方法 在这项 3 期试验中,新诊断为国际妇产科联合会 IC-IV 期上皮性卵巢癌的女性被随机分配到第 1 组(卡铂曲线下面积 [AUC]5 或 AUC6 和每 3 次 175 mg/m2 紫杉醇)周)、第 2 组(每 3 周卡铂 AUC5 或 AUC6 和每周 80 mg/m2 紫杉醇)或第 3 组(卡铂 AUC2 和每周 80 mg/m2 紫杉醇)。所有参与试验的女性均提供了书面知情同意书。该方案得到了进行研究的国家相应的国家研究伦理委员会的批准。患者在立即初次手术后或在新辅助化疗之前以及随后计划的延迟初次手术之前进入试验。试验的共同主要结果是无进展生存期和总生存期。数据分析是在意向治疗的基础上进行的,并有动力检测无进展生存期的风险比为 0·75。主要比较是在对照组(第 1 组)和每个每周研究组(第 2 组和第 3 组)之间进行的。结果 2011年6月6日至2014年11月28日期间,1566名女性被随机分配接受治疗。 第 1 组中 72% (365) 完成了 6 个方案定义的治疗周期,第 2 组中 60% (305) 完成了 63% (322),第 3 组中完成了 63% (322),尽管 90% (454)、89% (454) 和85% (437) 分别完成了六个铂类化疗周期。每周治疗可实现紫杉醇剂量强化(第1组紫杉醇中位总剂量为1010 mg/m2;第2组为1233 mg/m2;第3组为1274 mg/m2)。截至 2017 年 2 月,1018 名 (65%) 患者出现疾病进展。任一周治疗方案均未观察到显着的无进展生存期增加(第 1 组的限制平均生存时间为 24·4 个月 [97·5% CI 23·0-26·0],24·9 个月 [24·0-25] ·9] 在第 2 组中,25·3 个月 [23·9-26·9] 在第 3 组中;中位无进展生存期 17·7 个月 [IQR 10·6-未达到] 在第 1 组中,20·8 个月第 2 组为 [11·9-59·0],第 3 组为 21·0 个月 [12·0-54·0];第 2 组 vs 第 1 组 p=0·35; =0·51)。尽管 3 级或 4 级毒性作用随着每周治疗而增加,但这些作用主要并不复杂。各组间发热性中性粒细胞减少症和感觉神经病的发生率相似。解释 每周剂量密集化疗可以成功地作为上皮性卵巢癌的一线治疗,但与主要欧洲人群中标准的每 3 周化疗相比,并不能显着改善无进展生存期。资助英国癌症研究中心、医学研究委员会、爱尔兰健康研究委员会、爱尔兰癌症协会、澳大利亚癌症协会。
更新日期:2019-12-06
中文翻译:

一线上皮性卵巢癌、输卵管癌或原发性腹膜癌治疗中的每周剂量密集化疗 (ICON8):来自 GCIG 3 期随机对照试验的原发性无进展生存分析结果。
背景 每 3 周施用卡铂和紫杉醇是上皮性卵巢癌的标准一线化疗。日本 JGOG3016 试验显示,剂量密集的每周紫杉醇和每 3 周卡铂可显着改善无进展生存期和总生存期。在这项研究中,我们的目的是在主要患有上皮性卵巢癌的欧洲人群中比较两种剂量密集每周化疗方案与标准每周三周化疗的疗效和安全性。方法 在这项 3 期试验中,新诊断为国际妇产科联合会 IC-IV 期上皮性卵巢癌的女性被随机分配到第 1 组(卡铂曲线下面积 [AUC]5 或 AUC6 和每 3 次 175 mg/m2 紫杉醇)周)、第 2 组(每 3 周卡铂 AUC5 或 AUC6 和每周 80 mg/m2 紫杉醇)或第 3 组(卡铂 AUC2 和每周 80 mg/m2 紫杉醇)。所有参与试验的女性均提供了书面知情同意书。该方案得到了进行研究的国家相应的国家研究伦理委员会的批准。患者在立即初次手术后或在新辅助化疗之前以及随后计划的延迟初次手术之前进入试验。试验的共同主要结果是无进展生存期和总生存期。数据分析是在意向治疗的基础上进行的,并有动力检测无进展生存期的风险比为 0·75。主要比较是在对照组(第 1 组)和每个每周研究组(第 2 组和第 3 组)之间进行的。结果 2011年6月6日至2014年11月28日期间,1566名女性被随机分配接受治疗。 第 1 组中 72% (365) 完成了 6 个方案定义的治疗周期,第 2 组中 60% (305) 完成了 63% (322),第 3 组中完成了 63% (322),尽管 90% (454)、89% (454) 和85% (437) 分别完成了六个铂类化疗周期。每周治疗可实现紫杉醇剂量强化(第1组紫杉醇中位总剂量为1010 mg/m2;第2组为1233 mg/m2;第3组为1274 mg/m2)。截至 2017 年 2 月,1018 名 (65%) 患者出现疾病进展。任一周治疗方案均未观察到显着的无进展生存期增加(第 1 组的限制平均生存时间为 24·4 个月 [97·5% CI 23·0-26·0],24·9 个月 [24·0-25] ·9] 在第 2 组中,25·3 个月 [23·9-26·9] 在第 3 组中;中位无进展生存期 17·7 个月 [IQR 10·6-未达到] 在第 1 组中,20·8 个月第 2 组为 [11·9-59·0],第 3 组为 21·0 个月 [12·0-54·0];第 2 组 vs 第 1 组 p=0·35; =0·51)。尽管 3 级或 4 级毒性作用随着每周治疗而增加,但这些作用主要并不复杂。各组间发热性中性粒细胞减少症和感觉神经病的发生率相似。解释 每周剂量密集化疗可以成功地作为上皮性卵巢癌的一线治疗,但与主要欧洲人群中标准的每 3 周化疗相比,并不能显着改善无进展生存期。资助英国癌症研究中心、医学研究委员会、爱尔兰健康研究委员会、爱尔兰癌症协会、澳大利亚癌症协会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号