当前位置:
X-MOL 学术
›
Cancer Treat. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Contextualizing pertuzumab approval in the treatment of HER2-positive breast cancer patients.
Cancer Treatment Reviews ( IF 9.6 ) Pub Date : 2019-11-29 , DOI: 10.1016/j.ctrv.2019.101944 Javier Cortés 1 , Eva Ciruelos 2 , José Pérez-García 3 , Joan Albanell 4 , Laura García-Estévez 5 , Manuel Ruiz-Borrego 6 , Ruth Espinosa 7 , Isabel Gallegos 8 , Santiago González 9 , Isabel Álvarez 10 , Antonio Llombart 11
Cancer Treatment Reviews ( IF 9.6 ) Pub Date : 2019-11-29 , DOI: 10.1016/j.ctrv.2019.101944 Javier Cortés 1 , Eva Ciruelos 2 , José Pérez-García 3 , Joan Albanell 4 , Laura García-Estévez 5 , Manuel Ruiz-Borrego 6 , Ruth Espinosa 7 , Isabel Gallegos 8 , Santiago González 9 , Isabel Álvarez 10 , Antonio Llombart 11
Affiliation
|
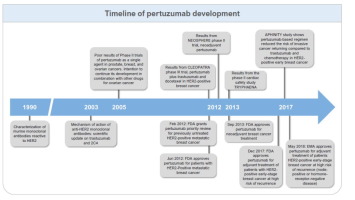
The use of adjuvant pertuzumab in HER2-positive early-stage breast cancer has recently been approved by the EMA on the basis of data from the APHINITY trial. Accordingly, we have produced this opinion article with the aim of putting the study data in perspective against other add-on therapeutic strategies, to clarify methodological or statistical doubts about the study, and to define the population of high-risk patients with hormone receptor-negative breast cancer that we agree, in general, should be treated. With this approval, physicians must be well prepared to place the APHINITY study data in context. It is now up to each country to ratify the EMA-approved indications and to agree on reimbursement, and doctors must optimize their use based on knowledge and discussion with patients.
中文翻译:

在治疗HER2阳性乳腺癌患者中将珀妥珠单抗的批准放到上下文中。
根据APHINITY试验的数据,EMA最近批准了佩妥珠单抗在HER2阳性早期乳腺癌中的使用。因此,我们撰写此评论文章的目的是将研究数据与其他附加治疗策略放在一起,以阐明对该研究的方法论或统计学疑问,并确定激素受体高危患者的人群。我们普遍认为阴性乳腺癌应该得到治疗。获得批准后,医生必须做好将APHINITY研究数据置于上下文中的准备。现在,每个国家都应批准EMA批准的适应症并同意报销,并且医生必须根据对患者的了解和讨论来优化其使用。
更新日期:2019-11-30
中文翻译:

在治疗HER2阳性乳腺癌患者中将珀妥珠单抗的批准放到上下文中。
根据APHINITY试验的数据,EMA最近批准了佩妥珠单抗在HER2阳性早期乳腺癌中的使用。因此,我们撰写此评论文章的目的是将研究数据与其他附加治疗策略放在一起,以阐明对该研究的方法论或统计学疑问,并确定激素受体高危患者的人群。我们普遍认为阴性乳腺癌应该得到治疗。获得批准后,医生必须做好将APHINITY研究数据置于上下文中的准备。现在,每个国家都应批准EMA批准的适应症并同意报销,并且医生必须根据对患者的了解和讨论来优化其使用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号