当前位置:
X-MOL 学术
›
npj Biofilms Microbiomes
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biofilms facilitate cheating and social exploitation of β-lactam resistance in Escherichia coli.
npj Biofilms and Microbiomes ( IF 7.8 ) Pub Date : 2019-11-29 , DOI: 10.1038/s41522-019-0109-2 Elli Amanatidou 1, 2 , Andrew C Matthews 3 , Ute Kuhlicke 4 , Thomas R Neu 4 , James P McEvoy 1 , Ben Raymond 1, 3
npj Biofilms and Microbiomes ( IF 7.8 ) Pub Date : 2019-11-29 , DOI: 10.1038/s41522-019-0109-2 Elli Amanatidou 1, 2 , Andrew C Matthews 3 , Ute Kuhlicke 4 , Thomas R Neu 4 , James P McEvoy 1 , Ben Raymond 1, 3
Affiliation
|
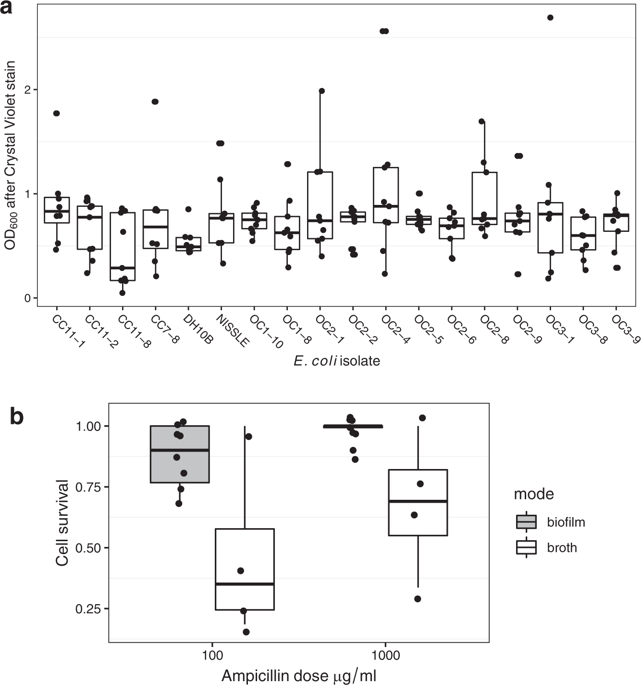
Gram-negative bacteria such as Escherichia coli commonly resist β-lactam antibiotics using plasmid-encoded β-lactamase enzymes. Bacterial strains that express β-lactamases have been found to detoxify liquid cultures and thus to protect genetically susceptible strains, constituting a clear laboratory example of social protection. These results are not necessarily general; on solid media, for instance, the rapid bactericidal action of β-lactams largely prevents social protection. Here, we tested the hypothesis that the greater tolerance of biofilm bacteria for β-lactams would facilitate social interactions. We used a recently isolated E. coli strain, capable of strong biofilm formation, to compare how cooperation and exploitation in colony biofilms and broth culture drives the dynamics of a non-conjugative plasmid encoding a clinically important β-lactamase. Susceptible cells in biofilms were tolerant of ampicillin-high doses and several days of exposure were required to kill them. In support of our hypothesis, we found robust social protection of susceptible E. coli in biofilms, despite fine-scale physical separation of resistant and susceptible cells and lower rates of production of extracellular β-lactamase. In contrast, social interactions in broth were restricted to a relatively narrow range of ampicillin doses. Our results show that β-lactam selection pressure on Gram-negative biofilms leads to cooperative resistance characterized by a low equilibrium frequency of resistance plasmids, sufficient to protect all cells.
中文翻译:

生物膜促进了大肠杆菌中β-内酰胺抗性的作弊和社会利用。
革兰氏阴性细菌(例如大肠杆菌)通常使用质粒编码的β-内酰胺酶来抵抗β-内酰胺类抗生素。已经发现表达β-内酰胺酶的细菌菌株可以使液体培养物解毒,从而保护遗传易感菌株,从而构成了社会保护的明确实验室实例。这些结果不一定是一般性的。例如,在固体培养基上,β-内酰胺的快速杀菌作用在很大程度上阻碍了社会保护。在这里,我们测试了以下假设:生物膜细菌对β-内酰胺的更大耐受性将促进社交互动。我们使用了最近分离的大肠杆菌菌株,该菌株具有很强的生物膜形成能力,比较菌落生物膜和肉汤培养中的合作和开发如何驱动编码临床上重要的β-内酰胺酶的非结合质粒的动力学。生物膜中的敏感细胞可以耐受高剂量的氨苄西林,因此需要暴露几天才能杀死它们。为了支持我们的假设,我们发现了生物膜中易感性大肠杆菌的强大社会保护,尽管耐药和易感细胞进行了大规模的物理分离,并且胞外β-内酰胺酶的产生率较低。相反,肉汤中的社交互动仅限于相对较窄范围的氨苄西林剂量。我们的结果表明,革兰氏阴性生物膜上的β-内酰胺选择压力会导致协同抗性,其特征是抗性质粒的平衡频率较低,足以保护所有细胞。
更新日期:2019-11-29
中文翻译:

生物膜促进了大肠杆菌中β-内酰胺抗性的作弊和社会利用。
革兰氏阴性细菌(例如大肠杆菌)通常使用质粒编码的β-内酰胺酶来抵抗β-内酰胺类抗生素。已经发现表达β-内酰胺酶的细菌菌株可以使液体培养物解毒,从而保护遗传易感菌株,从而构成了社会保护的明确实验室实例。这些结果不一定是一般性的。例如,在固体培养基上,β-内酰胺的快速杀菌作用在很大程度上阻碍了社会保护。在这里,我们测试了以下假设:生物膜细菌对β-内酰胺的更大耐受性将促进社交互动。我们使用了最近分离的大肠杆菌菌株,该菌株具有很强的生物膜形成能力,比较菌落生物膜和肉汤培养中的合作和开发如何驱动编码临床上重要的β-内酰胺酶的非结合质粒的动力学。生物膜中的敏感细胞可以耐受高剂量的氨苄西林,因此需要暴露几天才能杀死它们。为了支持我们的假设,我们发现了生物膜中易感性大肠杆菌的强大社会保护,尽管耐药和易感细胞进行了大规模的物理分离,并且胞外β-内酰胺酶的产生率较低。相反,肉汤中的社交互动仅限于相对较窄范围的氨苄西林剂量。我们的结果表明,革兰氏阴性生物膜上的β-内酰胺选择压力会导致协同抗性,其特征是抗性质粒的平衡频率较低,足以保护所有细胞。











































 京公网安备 11010802027423号
京公网安备 11010802027423号