当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of the cis/trans configuration on the supramolecular aggregation of aryltriazoles.
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2019-11-28 , DOI: 10.3762/bjoc.15.282 Sara Tejera 1 , Giada Caniglia 1 , Rosa L Dorta 1 , Andrea Favero 1 , Javier González-Platas 1 , Jesús T Vázquez 1
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2019-11-28 , DOI: 10.3762/bjoc.15.282 Sara Tejera 1 , Giada Caniglia 1 , Rosa L Dorta 1 , Andrea Favero 1 , Javier González-Platas 1 , Jesús T Vázquez 1
Affiliation
|
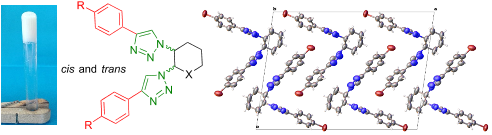
The ability of trans- and cis-1,2-glucopyranosyl and cyclohexyl ditriazoles, synthesized by CuAAC "click" chemistry, to form gels was studied, their physical properties determined, and the self-aggregation behavior investigated by SEM, X-ray, and EDC studies. The results revealed that self-assembly was driven mainly by π-π stacking interactions, in addition to hydrogen bonding, with the aromatic rings adopting a high degree of parallelism, as seen in crystal packings and ECD data. Furthermore, π-bromine interactions between the bromine atom of the aryl substituents and the triazole units might also contribute to an overall stabilization of the supramolecular aggregation of bis(4-bromophenyl)triazoles. The trans or cis spatial disposition of the triazole rings is highly important for gelation, with the cis configuration having higher propensity.
中文翻译:

顺式/反式构型对芳基三唑超分子聚集的影响。
研究了通过CuAAC“点击”化学合成的反式和顺式1,2-吡喃葡萄糖基和环己基二三唑形成凝胶的能力,确定了它们的物理性质,并通过SEM,X射线,和EDC研究。结果表明,自组装主要由π-π堆积相互作用驱动,除氢键作用外,芳环采用高度平行性,如晶体堆积和ECD数据所示。此外,芳基取代基的溴原子与三唑单元之间的π-溴相互作用也可能有助于双(4-溴苯基)三唑的超分子聚集的总体稳定。三唑环的反式或顺式空间位置对于胶凝非常重要,
更新日期:2019-11-29
中文翻译:

顺式/反式构型对芳基三唑超分子聚集的影响。
研究了通过CuAAC“点击”化学合成的反式和顺式1,2-吡喃葡萄糖基和环己基二三唑形成凝胶的能力,确定了它们的物理性质,并通过SEM,X射线,和EDC研究。结果表明,自组装主要由π-π堆积相互作用驱动,除氢键作用外,芳环采用高度平行性,如晶体堆积和ECD数据所示。此外,芳基取代基的溴原子与三唑单元之间的π-溴相互作用也可能有助于双(4-溴苯基)三唑的超分子聚集的总体稳定。三唑环的反式或顺式空间位置对于胶凝非常重要,











































 京公网安备 11010802027423号
京公网安备 11010802027423号