当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron‐Catalyzed Chemoselective C−N Coupling Reaction: A Protecting‐Group‐Free Amination of Aryl Halides Bearing Amino or Hydroxy Groups
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2019-12-04 , DOI: 10.1002/ajoc.201900641 Yuma Aoki 1, 2, 3 , Takahiro Toyoda 1, 2 , Hiroto Kawasaki 1, 2 , Hikaru Takaya 1, 2 , Akhilesh K. Sharma 1 , Keiji Morokuma 4 , Masaharu Nakamura 1, 2
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2019-12-04 , DOI: 10.1002/ajoc.201900641 Yuma Aoki 1, 2, 3 , Takahiro Toyoda 1, 2 , Hiroto Kawasaki 1, 2 , Hikaru Takaya 1, 2 , Akhilesh K. Sharma 1 , Keiji Morokuma 4 , Masaharu Nakamura 1, 2
Affiliation
|
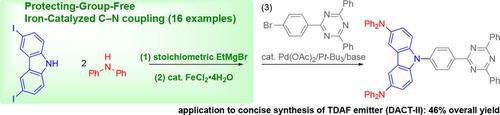
A chemoselective C−N coupling (Buchwald‐Hartwig‐type) reaction of diarylamines with aryl halides bearing non‐protected amino or hydroxy groups proceeds in the presence of a simple iron catalyst. Upon treatment with Grignard reagents, various diarylamines can be cross‐coupled with halocarbazoles, haloindoles, haloanilines, and halophenols to afford the corresponding triarylamines, without the undesired dimerization or oligomerization of the starting aryl halides. DFT studies on the dimeric iron amide intermediates reveal that the reductive elimination can be the selectivity determining step. Finally, a short‐step synthesis of a thermally activated delayed‐fluorescence emitter, DACT‐II, demonstrates the synthetic utility of the present method.
中文翻译:

铁催化的化学选择性CN偶联反应:带有氨基或羟基的芳基卤化物的无保护基胺化
在简单的铁催化剂存在下,二芳基胺与带有未保护氨基或羟基的芳基卤化物的化学选择性C-N偶联(Buchwald-Hartwig型)反应。用格氏试剂处理后,各种二芳基胺可与卤咔唑,卤吲哚,卤苯胺和卤酚交叉偶联,得到相应的三芳基胺,而不会发生不希望的起始卤代芳基二聚或低聚。对二聚铁酰胺中间体的DFT研究表明,还原消除可能是选择性的决定步骤。最后,热活化延迟荧光发射体DACT-II的短时合成证明了本方法的合成效用。
更新日期:2019-12-04
中文翻译:

铁催化的化学选择性CN偶联反应:带有氨基或羟基的芳基卤化物的无保护基胺化
在简单的铁催化剂存在下,二芳基胺与带有未保护氨基或羟基的芳基卤化物的化学选择性C-N偶联(Buchwald-Hartwig型)反应。用格氏试剂处理后,各种二芳基胺可与卤咔唑,卤吲哚,卤苯胺和卤酚交叉偶联,得到相应的三芳基胺,而不会发生不希望的起始卤代芳基二聚或低聚。对二聚铁酰胺中间体的DFT研究表明,还原消除可能是选择性的决定步骤。最后,热活化延迟荧光发射体DACT-II的短时合成证明了本方法的合成效用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号