当前位置:
X-MOL 学术
›
Nat. Protoc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Analyzing bacterial extracellular vesicles in human body fluids by orthogonal biophysical separation and biochemical characterization.
Nature Protocols ( IF 13.1 ) Pub Date : 2019-11-27 , DOI: 10.1038/s41596-019-0236-5 Joeri Tulkens 1, 2 , Olivier De Wever 1, 2 , An Hendrix 1, 2
Nature Protocols ( IF 13.1 ) Pub Date : 2019-11-27 , DOI: 10.1038/s41596-019-0236-5 Joeri Tulkens 1, 2 , Olivier De Wever 1, 2 , An Hendrix 1, 2
Affiliation
|
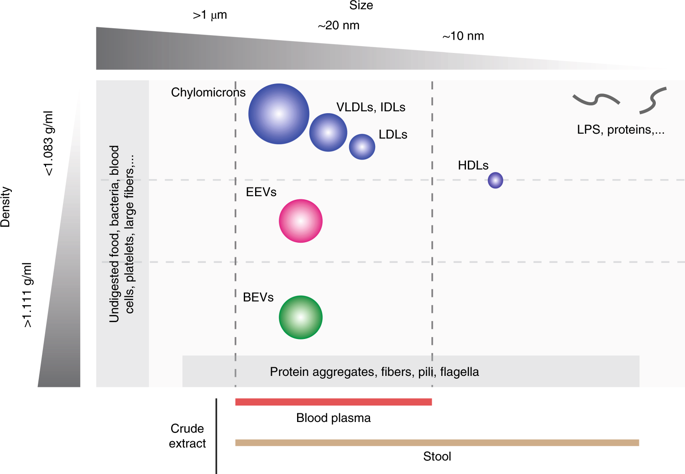
Gram-negative and Gram-positive bacteria release a variety of membrane vesicles through different formation routes. Knowledge of the structure, molecular cargo and function of bacterial extracellular vesicles (BEVs) is primarily obtained from bacteria cultured in laboratory conditions. BEVs in human body fluids have been less thoroughly investigated most probably due to the methodological challenges in separating BEVs from their matrix and host-derived eukaryotic extracellular vesicles (EEVs) such as exosomes and microvesicles. Here, we present a step-by-step procedure to separate and characterize BEVs from human body fluids. BEVs are separated through the orthogonal implementation of ultrafiltration, size-exclusion chromatography (SEC) and density-gradient centrifugation. Size separates BEVs from bacteria, flagella and cell debris in stool; and blood cells, high density lipoproteins (HDLs) and soluble proteins in blood. Density separates BEVs from fibers, protein aggregates and EEVs in stool; and low-density lipoproteins (LDLs), very-low-density lipoproteins (VLDLs), chylomicrons, protein aggregates and EEVs in blood. The procedure is label free, maintains the integrity of BEVs and ensures reproducibility through the use of automated liquid handlers. Post-separation BEVs are characterized using orthogonal biochemical endotoxin and Toll-like receptor-based reporter assays in combination with proteomics, electron microscopy and nanoparticle tracking analysis (NTA) to evaluate BEV quality, abundance, structure and molecular cargo. Separation and characterization of BEVs from body fluids can be done within 72 h, is compatible with EEV analysis and can be readily adopted by researchers experienced in basic molecular biology and extracellular vesicle analysis. We anticipate that this protocol will expand our knowledge on the biological heterogeneity, molecular cargo and function of BEVs in human body fluids and steer the development of laboratory research tools and clinical diagnostic kits.
中文翻译:

通过正交生物物理分离和生化表征分析人体液中的细菌细胞外囊泡。
革兰氏阴性和革兰氏阳性细菌通过不同的形成途径释放各种膜囊泡。细菌细胞外囊泡(BEV)的结构,分子物质和功能的知识主要来自在实验室条件下培养的细菌。人体液中的BEV尚未得到充分彻底的研究,这可能是由于将BEV从其基质和宿主衍生的真核细胞外囊泡(EEV)(如外来体和微囊泡)中分离出来的方法学上的挑战。在这里,我们介绍了从人体液中分离和表征BEV的分步过程。通过超滤,尺寸排阻色谱法(SEC)和密度梯度离心的正交实施分离BEV。大小可将BEV与粪便中的细菌,鞭毛和细胞碎片区分开;血液中的血细胞,高密度脂蛋白(HDL)和可溶性蛋白。密度可将BEV与粪便中的纤维,蛋白质聚集体和EEV分开;血液中的低密度脂蛋白(LDLs),超低密度脂蛋白(VLDLs),乳糜微粒,蛋白聚集物和EEV。该程序无标签,可保持BEV的完整性,并通过使用自动液体处理机来确保可重复性。分离后的BEV使用正交生化内毒素和基于Toll样受体的报道分子检测结合蛋白质组学,电子显微镜和纳米颗粒跟踪分析(NTA)进行表征,以评估BEV的质量,丰度,结构和分子货物。BEV与体液的分离和表征可在72小时内完成,与EEV分析兼容,并且具有基础分子生物学和细胞外囊泡分析经验的研究人员可以轻松采用。我们预计,该协议将扩大我们对BEV在人体体液中的生物学异质性,分子负载和功能的了解,并指导实验室研究工具和临床诊断试剂盒的开发。
更新日期:2019-11-28
中文翻译:

通过正交生物物理分离和生化表征分析人体液中的细菌细胞外囊泡。
革兰氏阴性和革兰氏阳性细菌通过不同的形成途径释放各种膜囊泡。细菌细胞外囊泡(BEV)的结构,分子物质和功能的知识主要来自在实验室条件下培养的细菌。人体液中的BEV尚未得到充分彻底的研究,这可能是由于将BEV从其基质和宿主衍生的真核细胞外囊泡(EEV)(如外来体和微囊泡)中分离出来的方法学上的挑战。在这里,我们介绍了从人体液中分离和表征BEV的分步过程。通过超滤,尺寸排阻色谱法(SEC)和密度梯度离心的正交实施分离BEV。大小可将BEV与粪便中的细菌,鞭毛和细胞碎片区分开;血液中的血细胞,高密度脂蛋白(HDL)和可溶性蛋白。密度可将BEV与粪便中的纤维,蛋白质聚集体和EEV分开;血液中的低密度脂蛋白(LDLs),超低密度脂蛋白(VLDLs),乳糜微粒,蛋白聚集物和EEV。该程序无标签,可保持BEV的完整性,并通过使用自动液体处理机来确保可重复性。分离后的BEV使用正交生化内毒素和基于Toll样受体的报道分子检测结合蛋白质组学,电子显微镜和纳米颗粒跟踪分析(NTA)进行表征,以评估BEV的质量,丰度,结构和分子货物。BEV与体液的分离和表征可在72小时内完成,与EEV分析兼容,并且具有基础分子生物学和细胞外囊泡分析经验的研究人员可以轻松采用。我们预计,该协议将扩大我们对BEV在人体体液中的生物学异质性,分子负载和功能的了解,并指导实验室研究工具和临床诊断试剂盒的开发。











































 京公网安备 11010802027423号
京公网安备 11010802027423号