当前位置:
X-MOL 学术
›
JAMA Oncol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sequential Ipilimumab After Chemoradiotherapy in Curative-Intent Treatment of Patients With Node-Positive Cervical Cancer.
JAMA Oncology ( IF 22.5 ) Pub Date : 2019-11-27 , DOI: 10.1001/jamaoncol.2019.3857 Jyoti S Mayadev 1 , Danielle Enserro 2 , Yvonne G Lin 3 , Diane M Da Silva 3 , Heather A Lankes 4 , Carol Aghajanian 5 , Sharad Ghamande 6 , Kathleen N Moore 7 , Vanessa A Kennedy 8 , Paula M Fracasso 9 , Russell J Schilder 10
JAMA Oncology ( IF 22.5 ) Pub Date : 2019-11-27 , DOI: 10.1001/jamaoncol.2019.3857 Jyoti S Mayadev 1 , Danielle Enserro 2 , Yvonne G Lin 3 , Diane M Da Silva 3 , Heather A Lankes 4 , Carol Aghajanian 5 , Sharad Ghamande 6 , Kathleen N Moore 7 , Vanessa A Kennedy 8 , Paula M Fracasso 9 , Russell J Schilder 10
Affiliation
|
Importance
Despite standard chemoradiotherapy (CRT), most women with lymph node (LN)-positive cervical cancer experience disease recurrence. Immunotherapy is being investigated in the up-front treatment setting.
Objectives
To assess the safety of sequential immunotherapy after CRT and to investigate human papillomavirus (HPV) genotype and HLA allele status on survival and programmed cell death 1 (PD-1) expression before and after CRT and sequential immunotherapy.
Design, Setting, and Participants
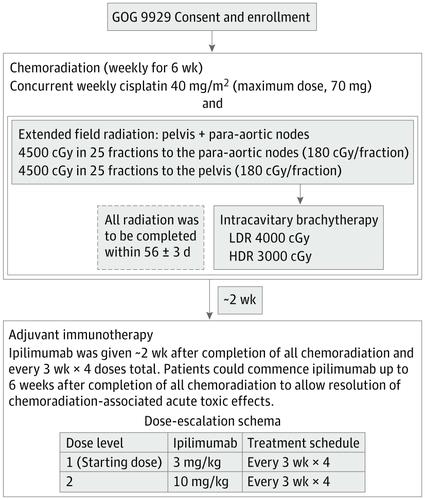
This prospective phase 1 trial conducted in 29 Gynecology Oncology Cooperative Group member institutions enrolled participants from December 18, 2012, to August 31, 2016, with a 14.8-month median follow-up and translational end points. Thirty-four women with International Federation of Gynecology and Obstetrics stage IB2 to IVA cervical cancer with positive pelvic LNs, para-aortic LNs, or both were enrolled; 13 did not receive ipilimumab and were excluded from the analysis. Data were analyzed from January 21 to April 4, 2018.
Interventions
Treatment consisted of 6 weekly doses of cisplatin, 40 mg/m2, concurrent with radiotherapy. After completion of chemotherapy, sequential ipilimumab was given every 21 days for 4 doses. Two dosage levels of ipilimumab, 3 mg/kg and 10 mg/kg, were studied to identify the maximum tolerated dose.
Main Outcomes and Measures
The primary end point was safety, and the secondary end points were overall survival and progression-free survival. Exploratory end points included HPV genotype, HLA allele status, and PD-1 expression measured in peripheral blood.
Results
The median age of the 32 participants included in the intent-to-treat analysis was 50 (range, 26-61) years, and 22 patients (69%) were white. Of the 21 patients who received ipilimumab, all had positive pelvic LN, and 6 (29%) had positive para-aortic LNs. All patients completed CRT, and of the 21 patients who received at least 2 cycles of ipilimumab, 18 (86%) completed 4 cycles of ipilimumab, and 3 (14%) completed 2 cycles. The maximum tolerated dose was 10 mg/kg. Two of the 21 patients (9.5%) who received ipilimumab had self-limiting grade 3 toxic effects (lipase increase; dermatitis). The 12-month overall survival was 90%, and progression-free survival was 81%. Human papillomavirus genotype and HLA subtype were not associated with progression-free survival or overall survival. T cells expressing PD-1 increased after CRT, and levels were sustained with ipilimumab.
Conclusions and Relevance
This study's findings suggest that the use of immunotherapy after CRT for curative-intent treatment of patients with cervical cancer is tolerable and effective. The results indicated that PD-1 was upregulated after CRT and sustained with sequential ipilimumab therapy. These immune findings may help guide future therapies to harness the activated T-cell phenotype in patients with node-positive cervical cancer.
中文翻译:

放化疗后序贯伊匹单抗对淋巴结阳性宫颈癌患者的根治性治疗。
重要性 尽管有标准的放化疗 (CRT),但大多数淋巴结 (LN) 阳性宫颈癌的女性都会出现疾病复发。免疫疗法正在前期治疗环境中进行研究。目的 评估 CRT 后序贯免疫治疗的安全性,并研究 CRT 和序贯免疫治疗前后人乳头瘤病毒 (HPV) 基因型和 HLA 等位基因状态对生存和程序性细胞死亡 1 (PD-1) 表达的影响。设计、设置和参与者 这项前瞻性 1 期试验于 2012 年 12 月 18 日至 2016 年 8 月 31 日在 29 家妇科肿瘤合作组成员机构中进行,招募参与者,中位随访时间为 14.8 个月,转化终点为 14.8 个月。纳入了 34 名国际妇产科联合会 IB2 至 IVA 宫颈癌且盆腔淋巴结阳性、主动脉旁淋巴结阳性或两者兼有的女性;13 名未接受易普利姆玛治疗并被排除在分析之外。数据分析时间为 2018 年 1 月 21 日至 4 月 4 日。干预治疗包括每周 6 剂 40 mg/m2 顺铂,同时进行放疗。化疗完成后,每 21 天连续给予 ipilimumab 4 剂。研究了易普利姆玛的两个剂量水平,3 mg/kg 和 10 mg/kg,以确定最大耐受剂量。主要结局指标主要终点是安全性,次要终点是总生存期和无进展生存期。探索性终点包括 HPV 基因型、HLA 等位基因状态、和在外周血中测量的 PD-1 表达。结果 意向治疗分析中纳入的 32 名参与者的中位年龄为 50(范围,26-61)岁,其中 22 名患者(69%)为白人。在接受易普利姆玛治疗的 21 名患者中,所有患者的盆腔 LN 均呈阳性,6 名(29%)的主动脉旁 LN 呈阳性。所有患者都完成了 CRT,在接受至少 2 个周期的易普利姆玛治疗的 21 名患者中,18 名(86%)完成了 4 个周期的易普利姆玛治疗,3 名(14%)完成了 2 个周期。最大耐受剂量为 10 mg/kg。接受易普利姆玛治疗的 21 名患者中有 2 名 (9.5%) 具有自限性 3 级毒性作用(脂肪酶增加;皮炎)。12个月总生存率为90%,无进展生存率为81%。人乳头瘤病毒基因型和 HLA 亚型与无进展生存期或总生存期无关。CRT 后表达 PD-1 的 T 细胞增加,而 ipilimumab 维持水平。结论和相关性 本研究的结果表明,在 CRT 后使用免疫疗法对宫颈癌患者进行根治性治疗是可耐受且有效的。结果表明,PD-1 在 CRT 后上调,并在连续 ipilimumab 治疗后持续。这些免疫发现可能有助于指导未来的治疗,以利用淋巴结阳性宫颈癌患者的活化 T 细胞表型。结果表明,PD-1 在 CRT 后上调,并在连续 ipilimumab 治疗后持续。这些免疫发现可能有助于指导未来的治疗,以利用淋巴结阳性宫颈癌患者的活化 T 细胞表型。结果表明,PD-1 在 CRT 后上调,并在连续 ipilimumab 治疗后持续。这些免疫发现可能有助于指导未来的治疗,以利用淋巴结阳性宫颈癌患者的活化 T 细胞表型。
更新日期:2020-01-09
中文翻译:

放化疗后序贯伊匹单抗对淋巴结阳性宫颈癌患者的根治性治疗。
重要性 尽管有标准的放化疗 (CRT),但大多数淋巴结 (LN) 阳性宫颈癌的女性都会出现疾病复发。免疫疗法正在前期治疗环境中进行研究。目的 评估 CRT 后序贯免疫治疗的安全性,并研究 CRT 和序贯免疫治疗前后人乳头瘤病毒 (HPV) 基因型和 HLA 等位基因状态对生存和程序性细胞死亡 1 (PD-1) 表达的影响。设计、设置和参与者 这项前瞻性 1 期试验于 2012 年 12 月 18 日至 2016 年 8 月 31 日在 29 家妇科肿瘤合作组成员机构中进行,招募参与者,中位随访时间为 14.8 个月,转化终点为 14.8 个月。纳入了 34 名国际妇产科联合会 IB2 至 IVA 宫颈癌且盆腔淋巴结阳性、主动脉旁淋巴结阳性或两者兼有的女性;13 名未接受易普利姆玛治疗并被排除在分析之外。数据分析时间为 2018 年 1 月 21 日至 4 月 4 日。干预治疗包括每周 6 剂 40 mg/m2 顺铂,同时进行放疗。化疗完成后,每 21 天连续给予 ipilimumab 4 剂。研究了易普利姆玛的两个剂量水平,3 mg/kg 和 10 mg/kg,以确定最大耐受剂量。主要结局指标主要终点是安全性,次要终点是总生存期和无进展生存期。探索性终点包括 HPV 基因型、HLA 等位基因状态、和在外周血中测量的 PD-1 表达。结果 意向治疗分析中纳入的 32 名参与者的中位年龄为 50(范围,26-61)岁,其中 22 名患者(69%)为白人。在接受易普利姆玛治疗的 21 名患者中,所有患者的盆腔 LN 均呈阳性,6 名(29%)的主动脉旁 LN 呈阳性。所有患者都完成了 CRT,在接受至少 2 个周期的易普利姆玛治疗的 21 名患者中,18 名(86%)完成了 4 个周期的易普利姆玛治疗,3 名(14%)完成了 2 个周期。最大耐受剂量为 10 mg/kg。接受易普利姆玛治疗的 21 名患者中有 2 名 (9.5%) 具有自限性 3 级毒性作用(脂肪酶增加;皮炎)。12个月总生存率为90%,无进展生存率为81%。人乳头瘤病毒基因型和 HLA 亚型与无进展生存期或总生存期无关。CRT 后表达 PD-1 的 T 细胞增加,而 ipilimumab 维持水平。结论和相关性 本研究的结果表明,在 CRT 后使用免疫疗法对宫颈癌患者进行根治性治疗是可耐受且有效的。结果表明,PD-1 在 CRT 后上调,并在连续 ipilimumab 治疗后持续。这些免疫发现可能有助于指导未来的治疗,以利用淋巴结阳性宫颈癌患者的活化 T 细胞表型。结果表明,PD-1 在 CRT 后上调,并在连续 ipilimumab 治疗后持续。这些免疫发现可能有助于指导未来的治疗,以利用淋巴结阳性宫颈癌患者的活化 T 细胞表型。结果表明,PD-1 在 CRT 后上调,并在连续 ipilimumab 治疗后持续。这些免疫发现可能有助于指导未来的治疗,以利用淋巴结阳性宫颈癌患者的活化 T 细胞表型。









































 京公网安备 11010802027423号
京公网安备 11010802027423号