当前位置:
X-MOL 学术
›
Magn. Reson. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and structural elucidation of a phthalide analog using NMR analysis and DFT calculations
Magnetic Resonance in Chemistry ( IF 1.9 ) Pub Date : 2020-01-02 , DOI: 10.1002/mrc.4976 Bryan N S Pinto 1 , Milena G Teixeira 1 , Elson S Alvarenga 1
Magnetic Resonance in Chemistry ( IF 1.9 ) Pub Date : 2020-01-02 , DOI: 10.1002/mrc.4976 Bryan N S Pinto 1 , Milena G Teixeira 1 , Elson S Alvarenga 1
Affiliation
|
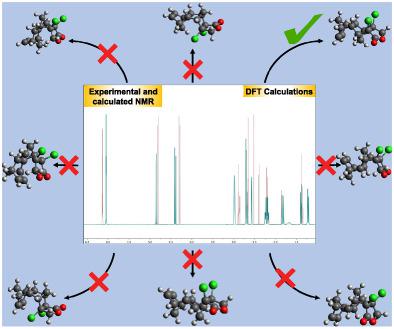
Phtalides are secondary metabolites found in several fungi with a wide range of biological activities. A novel phthalide analog was synthesized by Diels–Alder reaction between cyclopentadiene and 3,4‐dichlorofuran‐2(5H)‐one. Quantum mechanical calculations were used in conjunction with the spectrometric methods to determine the structure of the title compound. The calculated NMR chemical shifts for eight candidate pairs of enantiomers were compared with the experimental NMR chemical shifts applying the DP4 probability and mean absolute errors methodology. DP4 analysis using 1H and 13C NMR chemical shifts without assignment of the signals presented 100% probability for the correct candidate structure 3d, proving the consistency of the method even without spectra interpretation. Results from theoretical calculation and NMR spectra interpretation were in agreement to the structure of rac‐(3aR,4S,4aS,5R,8S,8aR,9R,9aS)‐3a,9a‐dichloro‐3a,4,4a,5,8,8a,9,9a‐octahydro‐4,9:5,8‐dimethanonaphtho[2,3‐c]furan‐1(3H)‐one.
中文翻译:

使用 NMR 分析和 DFT 计算合成和结构解析邻苯二甲酸酯类似物
邻苯二甲酸酯是在多种真菌中发现的次级代谢物,具有广泛的生物活性。通过环戊二烯和 3,4-二氯呋喃-2(5H)-one 之间的 Diels-Alder 反应合成了一种新型的邻苯二甲酸酯类似物。结合光谱方法使用量子力学计算来确定标题化合物的结构。将八对候选对映异构体的计算 NMR 化学位移与应用 DP4 概率和平均绝对误差方法的实验 NMR 化学位移进行比较。使用 1H 和 13C NMR 化学位移的 DP4 分析没有分配信号,显示正确的候选结构 3d 的概率为 100%,即使没有光谱解释也证明了该方法的一致性。
更新日期:2020-01-02
中文翻译:

使用 NMR 分析和 DFT 计算合成和结构解析邻苯二甲酸酯类似物
邻苯二甲酸酯是在多种真菌中发现的次级代谢物,具有广泛的生物活性。通过环戊二烯和 3,4-二氯呋喃-2(5H)-one 之间的 Diels-Alder 反应合成了一种新型的邻苯二甲酸酯类似物。结合光谱方法使用量子力学计算来确定标题化合物的结构。将八对候选对映异构体的计算 NMR 化学位移与应用 DP4 概率和平均绝对误差方法的实验 NMR 化学位移进行比较。使用 1H 和 13C NMR 化学位移的 DP4 分析没有分配信号,显示正确的候选结构 3d 的概率为 100%,即使没有光谱解释也证明了该方法的一致性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号