当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemoselective Tertiary C-H Hydroxylation for Late-Stage Functionalization with Mn(PDP)/Chloroacetic Acid Catalysis.
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-11-27 , DOI: 10.1002/adsc.201901472 Rachel K Chambers 1, 2 , Jinpeng Zhao 1, 2, 3 , Connor P Delaney 1 , M Christina White 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-11-27 , DOI: 10.1002/adsc.201901472 Rachel K Chambers 1, 2 , Jinpeng Zhao 1, 2, 3 , Connor P Delaney 1 , M Christina White 1
Affiliation
|
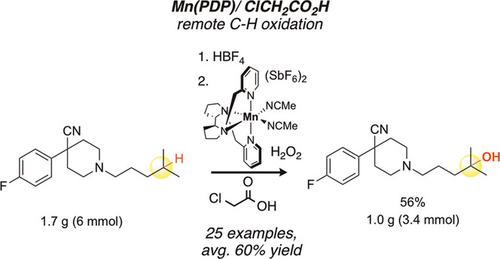
Aromatic and heterocyclic functionality are ubiquitous in pharmaceuticals. Herein, we disclose a new Mn(PDP)catalyst system using chloroacetic acid additive capable of chemoselectively oxidizing remote tertiary C(sp 3)-H bonds in the presence of a broad range of aromatic and heterocyclic moieties. Although catalyst loadings can be lowered to 0.1 mol% under a Mn(PDP)/acetic acid system for aromatic and non-basic nitrogen heterocycle substrates, the Mn(PDP)/chloroacetic acid system generally affords 10-15% higher isolated yields on these substrates and is uniquely effective for remote C(sp 3)-H hydroxylations in substrates housing basic nitrogen heterocycles. The demonstrated ability to perform Mn(PDP)/chloroacetic acid C(sp 3)-H oxidations in pharmaceutically relevant complex molecules on multi-gram scales will facilitate drug discovery processes via late-stage functionalization.
中文翻译:

化学选择性叔CH羟基化反应用于Mn(PDP)/氯乙酸催化的后期官能化。
芳香族和杂环官能团在药物中无处不在。在这里,我们公开了一种新的Mn(PDP)催化剂体系,它使用氯乙酸添加剂,能够在宽范围的芳族和杂环基团存在下化学选择性氧化远端C(sp 3)-H键。尽管对于芳香族和非碱性氮杂环底物,在Mn(PDP)/乙酸体系下催化剂负载量可降低至0.1 mol%,但Mn(PDP)/氯乙酸体系通常可将这些化合物的分离产率提高10-15%底物,并且对于容纳碱性氮杂环的底物中的远程C(sp 3)-H羟基化作用特别有效。
更新日期:2019-12-27
中文翻译:

化学选择性叔CH羟基化反应用于Mn(PDP)/氯乙酸催化的后期官能化。
芳香族和杂环官能团在药物中无处不在。在这里,我们公开了一种新的Mn(PDP)催化剂体系,它使用氯乙酸添加剂,能够在宽范围的芳族和杂环基团存在下化学选择性氧化远端C(sp 3)-H键。尽管对于芳香族和非碱性氮杂环底物,在Mn(PDP)/乙酸体系下催化剂负载量可降低至0.1 mol%,但Mn(PDP)/氯乙酸体系通常可将这些化合物的分离产率提高10-15%底物,并且对于容纳碱性氮杂环的底物中的远程C(sp 3)-H羟基化作用特别有效。











































 京公网安备 11010802027423号
京公网安备 11010802027423号