当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural and Biochemical Insight into the Recruitment of Acyl Carrier Protein-Linked Extender Units in Ansamitocin Biosynthesis.
ChemBioChem ( IF 2.6 ) Pub Date : 2020-01-10 , DOI: 10.1002/cbic.201900628 Fa Zhang 1 , Huining Ji 1 , Imtiaz Ali 1 , Zixin Deng 1 , Linquan Bai 1 , Jianting Zheng 1
ChemBioChem ( IF 2.6 ) Pub Date : 2020-01-10 , DOI: 10.1002/cbic.201900628 Fa Zhang 1 , Huining Ji 1 , Imtiaz Ali 1 , Zixin Deng 1 , Linquan Bai 1 , Jianting Zheng 1
Affiliation
|
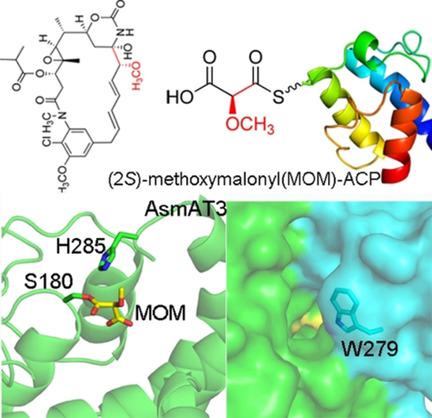
A few acyltransferase (AT) domains of modular polyketide synthases (PKSs) recruit acyl carrier protein (ACP)-linked extender units with unusual C2 substituents to confer functionalities that are not available in coenzyme A (CoA)-linked ones. In this study, an AT specific for methoxymalonyl (MOM)-ACP in the third module of the ansamitocin PKS was structurally and biochemically characterized. The AT uses a conserved tryptophan residue at the entrance of the substrate binding tunnel to discriminate between different carriers. A W275R mutation switches its carrier specificity from the ACP to the CoA molecule. The acyl-AT complex structures clearly show that the MOM-ACP accepted by the AT has the 2S instead of the opposite 2R stereochemistry that is predicted according to the biosynthetic derivation from a d-glycolytic intermediate. Together, these results reveal the structural basis of ATs recognizing ACP-linked extender units in polyketide biosynthesis.
中文翻译:

结构和生化方面的洞察力:安托霉素生物合成中酰基载体蛋白连接的延伸单元的募集。
模块化聚酮化合物合酶(PKS)的一些酰基转移酶(AT)域募集具有不常见C2取代基的酰基载体蛋白(ACP)连接的扩展单元,以赋予在辅酶A(CoA)连接中不可用的功能。在这项研究中,在结构上和生化上对安他霉素PKS第三模块中的甲氧基丙二酰(MOM)-ACP特有的AT进行了表征。AT在底物结合通道的入口使用保守的色氨酸残基来区分不同的载体。W275R突变将其载体特异性从ACP切换到CoA分子。酰基-AT复合物结构清楚地表明,AT接受的MOM-ACP具有2S而不是相反的2R立体化学,根据从d-糖酵解中间体的生物合成推导,该化学是预测的。一起,
更新日期:2020-01-10
中文翻译:

结构和生化方面的洞察力:安托霉素生物合成中酰基载体蛋白连接的延伸单元的募集。
模块化聚酮化合物合酶(PKS)的一些酰基转移酶(AT)域募集具有不常见C2取代基的酰基载体蛋白(ACP)连接的扩展单元,以赋予在辅酶A(CoA)连接中不可用的功能。在这项研究中,在结构上和生化上对安他霉素PKS第三模块中的甲氧基丙二酰(MOM)-ACP特有的AT进行了表征。AT在底物结合通道的入口使用保守的色氨酸残基来区分不同的载体。W275R突变将其载体特异性从ACP切换到CoA分子。酰基-AT复合物结构清楚地表明,AT接受的MOM-ACP具有2S而不是相反的2R立体化学,根据从d-糖酵解中间体的生物合成推导,该化学是预测的。一起,











































 京公网安备 11010802027423号
京公网安备 11010802027423号