当前位置:
X-MOL 学术
›
Lancet Oncol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A risk-based treatment strategy for non-rhabdomyosarcoma soft-tissue sarcomas in patients younger than 30 years (ARST0332): a Children's Oncology Group prospective study.
The Lancet Oncology ( IF 41.6 ) Pub Date : 2019-11-27 , DOI: 10.1016/s1470-2045(19)30672-2 Sheri L Spunt 1 , Lynn Million 2 , Yueh-Yun Chi 3 , James Anderson 4 , Jing Tian 3 , Emily Hibbitts 3 , Cheryl Coffin 5 , M Beth McCarville 6 , R Lor Randall 7 , David M Parham 8 , Jennifer O Black 9 , Simon C Kao 10 , Andrea Hayes-Jordan 11 , Suzanne Wolden 12 , Fran Laurie 13 , Roseanne Speights 14 , Ellen Kawashima 15 , Stephen X Skapek 16 , William Meyer 17 , Alberto S Pappo 14 , Douglas S Hawkins 18
The Lancet Oncology ( IF 41.6 ) Pub Date : 2019-11-27 , DOI: 10.1016/s1470-2045(19)30672-2 Sheri L Spunt 1 , Lynn Million 2 , Yueh-Yun Chi 3 , James Anderson 4 , Jing Tian 3 , Emily Hibbitts 3 , Cheryl Coffin 5 , M Beth McCarville 6 , R Lor Randall 7 , David M Parham 8 , Jennifer O Black 9 , Simon C Kao 10 , Andrea Hayes-Jordan 11 , Suzanne Wolden 12 , Fran Laurie 13 , Roseanne Speights 14 , Ellen Kawashima 15 , Stephen X Skapek 16 , William Meyer 17 , Alberto S Pappo 14 , Douglas S Hawkins 18
Affiliation
|
BACKGROUND
Tumour grade, tumour size, resection potential, and extent of disease affect outcome in paediatric non-rhabdomyosarcoma soft-tissue sarcoma (NRSTS), but no risk stratification systems exist and the standard of care is poorly defined. We developed a risk stratification system from known prognostic factors and assessed it in the context of risk-adapted therapy for young patients with NRSTS.
METHODS
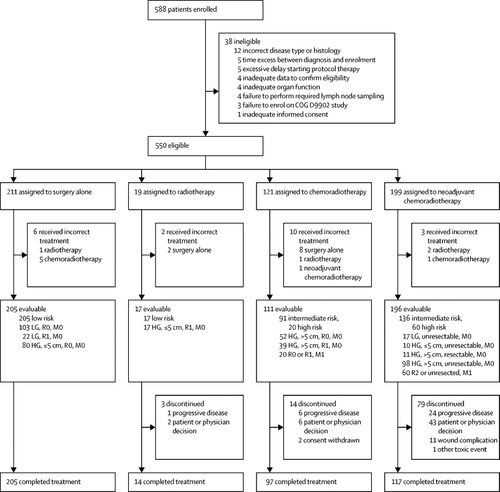
In this prospective study, eligible patients enrolled in 159 hospitals in three countries were younger than 30 years, had a Lansky (patients ≤16 years) or Karnofsky (patients >16 years) performance status score of at least 50, and a new diagnosis of a WHO (2002 criteria) intermediate (rarely metastasising) or malignant soft-tissue tumour (apart from tumour types eligible for other Children's Oncology Group studies and tumours for which the therapy in this trial was deemed inappropriate), malignant peripheral nerve sheath tumour, non-metastatic and grossly resected dermatofibrosarcoma protuberans, undifferentiated embryonal sarcoma of the liver, or unclassified malignant soft-tissue sarcoma. Each patient was assigned to one of three risk groups and one of four treatment groups. Risk groups were: low (non-metastatic R0 or R1 low-grade, or ≤5 cm R1 high-grade tumour); intermediate (non-metastatic R0 or R1 >5 cm high-grade, or unresected tumour of any size or grade); or high (metastatic tumour). The treatment groups were surgery alone, radiotherapy (55·8 Gy), chemoradiotherapy (chemotherapy and 55·8 Gy radiotherapy), and neoadjuvant chemoradiotherapy (chemotherapy and 45 Gy radiotherapy, then surgery and radiotherapy boost based on margins with continued chemotherapy). Chemotherapy included six cycles of ifosfamide 3 g/m2 per dose intravenously on days 1-3 and five cycles of doxorubicin 37·5 mg/m2 per dose intravenously on days 1-2 every 3 weeks with sequence adjusted on the basis of timing of surgery or radiotherapy. The primary outcomes were event-free survival, overall survival, and the pattern of treatment failure. Analysis was done per protocol. This study has been completed and is registered with ClinicalTrials.gov, NCT00346164.
FINDINGS
Between Feb 5, 2007, and Feb 10, 2012, 550 eligible patients were enrolled, of whom 21 were treated in the incorrect group and excluded from this analysis. 529 evaluable patients were included in the analysis: low-risk (n=222), intermediate-risk (n=227), high-risk (n=80); surgery alone (n=205), radiotherapy (n=17), chemoradiotherapy (n=111), and neoadjuvant chemoradiotherapy (n=196). At a median follow-up of 6·5 years (IQR 4·9-7·9), 5-year event-free survival and overall survival were: 88·9% (95% CI 84·0-93·8) and 96·2% (93·2-99·2) in the low-risk group; 65·0% (58·2-71·8) and 79·2% (73·4-85·0) in the intermediate-risk group; and 21·2% (11·4-31·1) and 35·5% (23·6-47·4) in the high-risk group, respectively. Risk group predicted event-free survival and overall survival (p<0·0001). No deaths from toxic events during treatment were reported. Nine patients had unexpected grade 4 adverse events (chemoradiotherapy group, n=2; neoadjuvant chemoradiotherapy group, n=7), including three wound complications that required surgery (all in the neoadjuvant chemoradiotherapy group).
INTERPRETATION
Pre-treatment clinical features can be used to effectively define treatment failure risk and to stratify young patients with NRSTS for risk-adapted therapy. Most low-risk patients can be cured without adjuvant therapy, thereby avoiding known long-term treatment complications. Survival remains suboptimal for intermediate-risk and high-risk patients and novel therapies are needed.
FUNDING
National Institutes of Health, St Baldrick's Foundation, Seattle Children's Foundation, American Lebanese Syrian Associated Charities.
中文翻译:

30 岁以下患者非横纹肌肉瘤软组织肉瘤的基于风险的治疗策略 (ARST0332):儿童肿瘤学组前瞻性研究。
背景 肿瘤分级、肿瘤大小、切除潜力和疾病范围会影响小儿非横纹肌肉瘤软组织肉瘤 (NRSTS) 的预后,但不存在风险分层系统,并且护理标准定义不明确。我们根据已知的预后因素开发了一个风险分层系统,并在针对年轻 NRSTS 患者的风险适应治疗的背景下对其进行了评估。方WHO(2002 年标准)中间(很少转移)或恶性软组织肿瘤(符合其他儿童的肿瘤类型除外)s 肿瘤学组研究和本试验中的治疗被认为不合适的肿瘤)、恶性周围神经鞘瘤、非转移性和大块切除的隆突性皮肤纤维肉瘤、未分化的肝胚胎性肉瘤或未分类的恶性软组织肉瘤。每位患者被分配到三个风险组之一和四个治疗组之一。风险组为:低(非转移性 R0 或 R1 低级别,或≤5 cm R1 高级别肿瘤);中间(非转移性 R0 或 R1 >5 cm 高级别,或任何大小或级别的未切除肿瘤);或高(转移性肿瘤)。治疗组为单纯手术、放疗(55·8 Gy)、放化疗(化疗+55·8 Gy放疗)、新辅助放化疗(化疗+45 Gy放疗,然后根据持续化疗的边缘进行手术和放疗增强)。化疗包括在第 1-3 天静脉注射 6 个周期的异环磷酰胺每剂 3 g/m2,在第 1-2 天每 3 周静脉注射 5 个周期的多柔比星 37·5 mg/m2 每剂,并根据手术时间调整顺序或放射治疗。主要结果是无事件生存期、总生存期和治疗失败的模式。根据方案进行分析。本研究已完成并在 ClinicalTrials.gov 注册,NCT00346164。结果 在 2007 年 2 月 5 日至 2012 年 2 月 10 日期间,共招募了 550 名符合条件的患者,其中 21 名患者接受了错误治疗,并被排除在该分析之外。529 名可评估患者被纳入分析:低风险 (n=222)、中风险 (n=227)、高风险 (n=80);单独手术(n=205)、放疗(n=17)、放化疗(n=111)和新辅助放化疗(n=196)。中位随访 6·5 年 (IQR 4·9-7·9),5 年无事件生存率和总生存率为:88·9% (95% CI 84·0-93·8)低风险组为 96·2% (93·2-99·2);中危组65·0%(58·2-71·8)和79·2%(73·4-85·0);高危组分别为 21·2% (11·4-31·1) 和 35·5% (23·6-47·4)。风险组预测无事件生存期和总生存期(p<0·0001)。没有报告治疗期间因毒性事件导致的死亡。9 例患者出现意外的 4 级不良事件(放化疗组,n=2;新辅助放化疗组,n=7),包括 3 例需要手术的伤口并发症(均在新辅助放化疗组)。解释 治疗前临床特征可用于有效定义治疗失败风险,并对年轻 NRSTS 患者进行风险适应治疗。大多数低风险患者无需辅助治疗即可治愈,从而避免已知的长期治疗并发症。中危和高危患者的生存仍然不是最理想的,需要新的治疗方法。资助美国国立卫生研究院、圣鲍德里克基金会、西雅图儿童基金会、美国黎巴嫩叙利亚联合慈善机构。中危和高危患者的生存仍然不是最理想的,需要新的治疗方法。资助美国国立卫生研究院、圣鲍德里克基金会、西雅图儿童基金会、美国黎巴嫩叙利亚联合慈善机构。中危和高危患者的生存仍然不是最理想的,需要新的治疗方法。资助美国国立卫生研究院、圣鲍德里克基金会、西雅图儿童基金会、美国黎巴嫩叙利亚联合慈善机构。
更新日期:2020-01-04
中文翻译:

30 岁以下患者非横纹肌肉瘤软组织肉瘤的基于风险的治疗策略 (ARST0332):儿童肿瘤学组前瞻性研究。
背景 肿瘤分级、肿瘤大小、切除潜力和疾病范围会影响小儿非横纹肌肉瘤软组织肉瘤 (NRSTS) 的预后,但不存在风险分层系统,并且护理标准定义不明确。我们根据已知的预后因素开发了一个风险分层系统,并在针对年轻 NRSTS 患者的风险适应治疗的背景下对其进行了评估。方WHO(2002 年标准)中间(很少转移)或恶性软组织肿瘤(符合其他儿童的肿瘤类型除外)s 肿瘤学组研究和本试验中的治疗被认为不合适的肿瘤)、恶性周围神经鞘瘤、非转移性和大块切除的隆突性皮肤纤维肉瘤、未分化的肝胚胎性肉瘤或未分类的恶性软组织肉瘤。每位患者被分配到三个风险组之一和四个治疗组之一。风险组为:低(非转移性 R0 或 R1 低级别,或≤5 cm R1 高级别肿瘤);中间(非转移性 R0 或 R1 >5 cm 高级别,或任何大小或级别的未切除肿瘤);或高(转移性肿瘤)。治疗组为单纯手术、放疗(55·8 Gy)、放化疗(化疗+55·8 Gy放疗)、新辅助放化疗(化疗+45 Gy放疗,然后根据持续化疗的边缘进行手术和放疗增强)。化疗包括在第 1-3 天静脉注射 6 个周期的异环磷酰胺每剂 3 g/m2,在第 1-2 天每 3 周静脉注射 5 个周期的多柔比星 37·5 mg/m2 每剂,并根据手术时间调整顺序或放射治疗。主要结果是无事件生存期、总生存期和治疗失败的模式。根据方案进行分析。本研究已完成并在 ClinicalTrials.gov 注册,NCT00346164。结果 在 2007 年 2 月 5 日至 2012 年 2 月 10 日期间,共招募了 550 名符合条件的患者,其中 21 名患者接受了错误治疗,并被排除在该分析之外。529 名可评估患者被纳入分析:低风险 (n=222)、中风险 (n=227)、高风险 (n=80);单独手术(n=205)、放疗(n=17)、放化疗(n=111)和新辅助放化疗(n=196)。中位随访 6·5 年 (IQR 4·9-7·9),5 年无事件生存率和总生存率为:88·9% (95% CI 84·0-93·8)低风险组为 96·2% (93·2-99·2);中危组65·0%(58·2-71·8)和79·2%(73·4-85·0);高危组分别为 21·2% (11·4-31·1) 和 35·5% (23·6-47·4)。风险组预测无事件生存期和总生存期(p<0·0001)。没有报告治疗期间因毒性事件导致的死亡。9 例患者出现意外的 4 级不良事件(放化疗组,n=2;新辅助放化疗组,n=7),包括 3 例需要手术的伤口并发症(均在新辅助放化疗组)。解释 治疗前临床特征可用于有效定义治疗失败风险,并对年轻 NRSTS 患者进行风险适应治疗。大多数低风险患者无需辅助治疗即可治愈,从而避免已知的长期治疗并发症。中危和高危患者的生存仍然不是最理想的,需要新的治疗方法。资助美国国立卫生研究院、圣鲍德里克基金会、西雅图儿童基金会、美国黎巴嫩叙利亚联合慈善机构。中危和高危患者的生存仍然不是最理想的,需要新的治疗方法。资助美国国立卫生研究院、圣鲍德里克基金会、西雅图儿童基金会、美国黎巴嫩叙利亚联合慈善机构。中危和高危患者的生存仍然不是最理想的,需要新的治疗方法。资助美国国立卫生研究院、圣鲍德里克基金会、西雅图儿童基金会、美国黎巴嫩叙利亚联合慈善机构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号