当前位置:
X-MOL 学术
›
Lancet Oncol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial.
The Lancet Oncology ( IF 41.6 ) Pub Date : 2019-11-27 , DOI: 10.1016/s1470-2045(19)30689-8 Peter Schmid 1 , Hope S Rugo 2 , Sylvia Adams 3 , Andreas Schneeweiss 4 , Carlos H Barrios 5 , Hiroji Iwata 6 , Véronique Diéras 7 , Volkmar Henschel 8 , Luciana Molinero 9 , Stephen Y Chui 9 , Vidya Maiya 9 , Amreen Husain 8 , Eric P Winer 10 , Sherene Loi 11 , Leisha A Emens 12 ,
The Lancet Oncology ( IF 41.6 ) Pub Date : 2019-11-27 , DOI: 10.1016/s1470-2045(19)30689-8 Peter Schmid 1 , Hope S Rugo 2 , Sylvia Adams 3 , Andreas Schneeweiss 4 , Carlos H Barrios 5 , Hiroji Iwata 6 , Véronique Diéras 7 , Volkmar Henschel 8 , Luciana Molinero 9 , Stephen Y Chui 9 , Vidya Maiya 9 , Amreen Husain 8 , Eric P Winer 10 , Sherene Loi 11 , Leisha A Emens 12 ,
Affiliation
|
BACKGROUND
Immunotherapy in combination with chemotherapy has shown promising efficacy across many different tumour types. We report the prespecified second interim overall survival analysis of the phase 3 IMpassion130 study assessing the efficacy and safety of atezolizumab plus nab-paclitaxel in patients with unresectable, locally advanced or metastatic triple-negative breast cancer.
METHODS
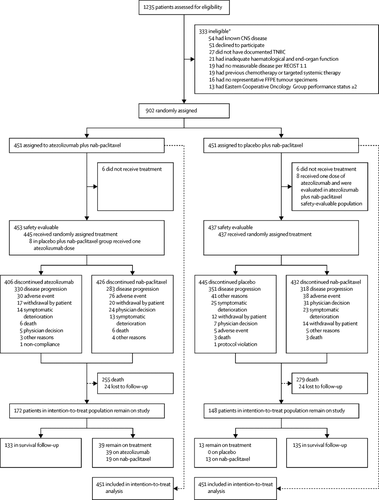
In this randomised, placebo-controlled, double-blind, phase 3 trial, done in 246 academic centres and community oncology practices in 41 countries, patients aged 18 years or older, with previously untreated, histologically documented, locally advanced or metastatic triple-negative breast cancer, and Eastern Cooperative Oncology Group performance status of 0 or 1 were eligible. Patients were randomly assigned (1:1) using a permuted block method (block size of four) and an interactive voice-web response system. Randomisation was stratified by previous taxane use, liver metastases, and PD-L1 expression on tumour-infiltrating immune cells. Patients received atezolizumab 840 mg or matching placebo intravenously on day 1 and day 15 of every 28-day cycle and nab-paclitaxel 100 mg/m2 of body surface area intravenously on days 1, 8, and 15 until progression or unacceptable toxicity. Investigators, patients, and the funder were masked to treatment assignment. Coprimary endpoints were investigator-assessed progression-free survival per Response Evaluation Criteria in Solid Tumors version 1.1 and overall survival, assessed in the intention-to-treat population and in patients with PD-L1 immune cell-positive tumours (tumours with ≥1% PD-L1 expression). The final progression-free survival results were previously reported at the first interim overall survival analysis. The prespecified statistical testing hierarchy meant that overall survival in the subgroup of PD-L1 immune cell-positive patients could only be formally tested if overall survival was significantly different between the treatment groups in the intention-to-treat population. This study is registered with ClinicalTrials.gov, NCT02425891.
FINDINGS
Between June 23, 2015, and May 24, 2017, 902 patients were enrolled, of whom 451 were randomly assigned to receive atezolizumab plus nab-paclitaxel and 451 were assigned to receive placebo plus nab-paclitaxel (the intention-to-treat population). Six patients from each group did not receive treatment. At the second interim analysis (data cutoff Jan 2, 2019), median follow-up was 18·5 months (IQR 9·6-22·8) in the atezolizumab group and 17·5 months (8·4-22·4) in the placebo group. Median overall survival in the intention-to-treat patients was 21·0 months (95% CI 19·0-22·6) with atezolizumab and 18·7 months (16·9-20·3) with placebo (stratified hazard ratio [HR] 0·86, 95% CI 0·72-1·02, p=0·078). In the exploratory overall survival analysis in patients with PD-L1 immune cell-positive tumours, median overall survival was 25·0 months (95% CI 19·6-30·7) with atezolizumab versus 18·0 months (13·6-20·1) with placebo (stratified HR 0·71, 0·54-0·94]). As of Sept 3, 2018 (the date up to which updated safety data were available), the most common grade 3-4 adverse events were neutropenia (38 [8%] of 453 patients in the atezolizumab group vs 36 [8%] of 437 patients in the placebo group), peripheral neuropathy (25 [6%] vs 12 [3%]), decreased neutrophil count (22 [5%] vs 16 [4%]), and fatigue (17 [4%] vs 15 [3%]). Treatment-related deaths occurred in two (<1%) patients in the atezolizumab group (autoimmune hepatitis related to atezolizumab [n=1] and septic shock related to nab-paclitaxel [n=1]) and one (<1%) patient in the placebo group (hepatic failure). No new treatment-related deaths have been reported since the primary clinical data cutoff date (April 17, 2018).
INTERPRETATION
Consistent with the first interim analysis, this second interim overall survival analysis of IMpassion130 indicates no significant difference in overall survival between the treatment groups in the intention-to-treat population but suggests a clinically meaningful overall survival benefit with atezolizumab plus nab-paclitaxel in patients with PD-L1 immune cell-positive disease. However, this positive result could not be formally tested due to the prespecified statistical testing hierarchy. For patients with PD-L1 immune cell-positive metastatic triple-negative breast cancer, atezolizumab plus nab-paclitaxel is an important therapeutic option in a disease with high unmet need.
FUNDING
F Hoffmann-La Roche and Genentech.
中文翻译:

Atezolizumab联合nab-紫杉醇作为不可切除,局部晚期或转移性三阴性乳腺癌(IMpassion130)的一线治疗:来自一项随机,双盲,安慰剂对照的3期试验的最新疗效结果。
背景技术免疫疗法与化学疗法相结合已经显示出在许多不同肿瘤类型上的有希望的功效。我们报告了3期IMpassion130研究的预先确定的第二次总体生存期分析,该研究评估了atezolizumab联合nab-紫杉醇在不可切除,局部晚期或转移性三阴性乳腺癌患者中的疗效和安全性。方法在这项随机,安慰剂对照,双盲,3期临床试验中,该试验在41个国家/地区的246个学术中心和社区肿瘤学实践中进行,年龄在18岁以上的患者以前未经治疗,组织学记录,局部晚期或转移性三联征。阴性乳腺癌和东部合作肿瘤小组的表现状态为0或1是合格的。患者被随机分配(1:1)使用置换块方法(块大小为4)和交互式语音Web响应系统。先前的紫杉烷使用,肝转移和肿瘤浸润性免疫细胞上PD-L1的表达对随机分组进行了分层。在每个28天周期的第1天和第15天,患者静脉注射atezolizumab 840 mg或与之匹配的安慰剂,并在第1、8和15天静脉注射nab-紫杉醇100 mg / m2的体表面积,直至进展或出现不可接受的毒性。研究人员,患者和资助者都被掩盖了治疗任务。共同的主要终点指标是根据研究者评估的1.1版实体瘤反应评估标准评估的无进展生存期和总体生存期,在意向性治疗人群和PD-L1免疫细胞阳性肿瘤(≥1%肿瘤患者)中进行评估PD-L1表达)。最终的无进展生存期结果先前已在首次中期总体生存期分析中报告。预先指定的统计测试体系意味着PD-L1免疫细胞阳性患者亚组的总体生存率只有在意向性治疗人群中各治疗组之间的总体生存率显着不同时才能进行正式测试。该研究已在ClinicalTrials.gov注册,NCT02425891。结果在2015年6月23日至2017年5月24日之间,共招募902名患者,其中451名患者被随机分配接受阿泰珠单抗加纳布-紫杉醇治疗,而451名患者被接受安慰剂加纳布-紫杉醇治疗(意向治疗人群) )。每组六名患者未接受治疗。在第二次中期分析中(数据截止于2019年1月2日),Atezolizumab组的中位随访时间为18·5个月(IQR 9·6-22·8),安慰剂组为17·5个月(8·4-22·4)。有意治疗的患者的中位总生存期为接受阿佐珠单抗的21·0个月(95%CI 19·0-22·6)和接受安慰剂的18·7个月(16·9-20·3)(分层危险比) [HR] 0·86,95%CI 0·72-1·02,p = 0·078)。在探索性PD-L1免疫细胞阳性肿瘤患者的总生存期分析中,使用阿扎佐珠单抗的中位总生存期为25·0个月(95%CI 19·6-30·7),而中位总生存期为18·0个月(13·6- 20·1)和安慰剂(分层HR 0·71、0·54-0·94])。截至2018年9月3日(可获得最新安全性数据的日期),最常见的3-4级不良事件是中性粒细胞减少症(阿特珠单抗组453例患者中38例[8%],而36例8%的不良反应)。安慰剂组中的437名患者),周围神经病变(25 [6%] vs 12 [3%]),中性粒细胞减少(22 [5%] vs 16 [4%])和疲劳(17 [4%] vs 15 [3%])。Atezolizumab组中有2名(<1%)患者与治疗有关的死亡(与atezolizumab相关的自身免疫性肝炎[n = 1]和与nab-紫杉醇有关的败血症性休克[n = 1])和1名(<1%)患者在安慰剂组(肝功能衰竭)。自主要临床数据截止日期(2018年4月17日)以来,尚无新的治疗相关死亡的报道。解释与第一次中期分析一致,IMpassion130的第二次中期总体生存期分析表明,意向性治疗人群中治疗组之间的总体生存期无显着差异,但建议在使用PD-L1免疫细胞-阳性疾病。但是,由于预先指定的统计测试层次结构,因此无法正式测试此肯定结果。对于患有PD-L1免疫细胞阳性转移性三阴性乳腺癌的患者,阿特珠单抗联合nab-紫杉醇是严重未满足需求的疾病的重要治疗选择。资金筹集Hoffmann-La Roche和Genentech。对于PD-L1免疫细胞阳性转移性三阴性乳腺癌患者,阿特珠单抗联合nab-紫杉醇是需要量未得到满足的疾病的重要治疗选择。资金筹集Hoffmann-La Roche和Genentech。对于PD-L1免疫细胞阳性转移性三阴性乳腺癌患者,阿特珠单抗联合nab-紫杉醇是需要量未得到满足的疾病的重要治疗选择。资金筹集Hoffmann-La Roche和Genentech。
更新日期:2020-01-04
中文翻译:

Atezolizumab联合nab-紫杉醇作为不可切除,局部晚期或转移性三阴性乳腺癌(IMpassion130)的一线治疗:来自一项随机,双盲,安慰剂对照的3期试验的最新疗效结果。
背景技术免疫疗法与化学疗法相结合已经显示出在许多不同肿瘤类型上的有希望的功效。我们报告了3期IMpassion130研究的预先确定的第二次总体生存期分析,该研究评估了atezolizumab联合nab-紫杉醇在不可切除,局部晚期或转移性三阴性乳腺癌患者中的疗效和安全性。方法在这项随机,安慰剂对照,双盲,3期临床试验中,该试验在41个国家/地区的246个学术中心和社区肿瘤学实践中进行,年龄在18岁以上的患者以前未经治疗,组织学记录,局部晚期或转移性三联征。阴性乳腺癌和东部合作肿瘤小组的表现状态为0或1是合格的。患者被随机分配(1:1)使用置换块方法(块大小为4)和交互式语音Web响应系统。先前的紫杉烷使用,肝转移和肿瘤浸润性免疫细胞上PD-L1的表达对随机分组进行了分层。在每个28天周期的第1天和第15天,患者静脉注射atezolizumab 840 mg或与之匹配的安慰剂,并在第1、8和15天静脉注射nab-紫杉醇100 mg / m2的体表面积,直至进展或出现不可接受的毒性。研究人员,患者和资助者都被掩盖了治疗任务。共同的主要终点指标是根据研究者评估的1.1版实体瘤反应评估标准评估的无进展生存期和总体生存期,在意向性治疗人群和PD-L1免疫细胞阳性肿瘤(≥1%肿瘤患者)中进行评估PD-L1表达)。最终的无进展生存期结果先前已在首次中期总体生存期分析中报告。预先指定的统计测试体系意味着PD-L1免疫细胞阳性患者亚组的总体生存率只有在意向性治疗人群中各治疗组之间的总体生存率显着不同时才能进行正式测试。该研究已在ClinicalTrials.gov注册,NCT02425891。结果在2015年6月23日至2017年5月24日之间,共招募902名患者,其中451名患者被随机分配接受阿泰珠单抗加纳布-紫杉醇治疗,而451名患者被接受安慰剂加纳布-紫杉醇治疗(意向治疗人群) )。每组六名患者未接受治疗。在第二次中期分析中(数据截止于2019年1月2日),Atezolizumab组的中位随访时间为18·5个月(IQR 9·6-22·8),安慰剂组为17·5个月(8·4-22·4)。有意治疗的患者的中位总生存期为接受阿佐珠单抗的21·0个月(95%CI 19·0-22·6)和接受安慰剂的18·7个月(16·9-20·3)(分层危险比) [HR] 0·86,95%CI 0·72-1·02,p = 0·078)。在探索性PD-L1免疫细胞阳性肿瘤患者的总生存期分析中,使用阿扎佐珠单抗的中位总生存期为25·0个月(95%CI 19·6-30·7),而中位总生存期为18·0个月(13·6- 20·1)和安慰剂(分层HR 0·71、0·54-0·94])。截至2018年9月3日(可获得最新安全性数据的日期),最常见的3-4级不良事件是中性粒细胞减少症(阿特珠单抗组453例患者中38例[8%],而36例8%的不良反应)。安慰剂组中的437名患者),周围神经病变(25 [6%] vs 12 [3%]),中性粒细胞减少(22 [5%] vs 16 [4%])和疲劳(17 [4%] vs 15 [3%])。Atezolizumab组中有2名(<1%)患者与治疗有关的死亡(与atezolizumab相关的自身免疫性肝炎[n = 1]和与nab-紫杉醇有关的败血症性休克[n = 1])和1名(<1%)患者在安慰剂组(肝功能衰竭)。自主要临床数据截止日期(2018年4月17日)以来,尚无新的治疗相关死亡的报道。解释与第一次中期分析一致,IMpassion130的第二次中期总体生存期分析表明,意向性治疗人群中治疗组之间的总体生存期无显着差异,但建议在使用PD-L1免疫细胞-阳性疾病。但是,由于预先指定的统计测试层次结构,因此无法正式测试此肯定结果。对于患有PD-L1免疫细胞阳性转移性三阴性乳腺癌的患者,阿特珠单抗联合nab-紫杉醇是严重未满足需求的疾病的重要治疗选择。资金筹集Hoffmann-La Roche和Genentech。对于PD-L1免疫细胞阳性转移性三阴性乳腺癌患者,阿特珠单抗联合nab-紫杉醇是需要量未得到满足的疾病的重要治疗选择。资金筹集Hoffmann-La Roche和Genentech。对于PD-L1免疫细胞阳性转移性三阴性乳腺癌患者,阿特珠单抗联合nab-紫杉醇是需要量未得到满足的疾病的重要治疗选择。资金筹集Hoffmann-La Roche和Genentech。











































 京公网安备 11010802027423号
京公网安备 11010802027423号