Chemical Physics ( IF 2.0 ) Pub Date : 2019-11-27 , DOI: 10.1016/j.chemphys.2019.110641 Bao-Li Wang , Dong-Qi Pan , Song-Bo Kou , Zhen-Yi Lin , Jie-Hua Shi
|
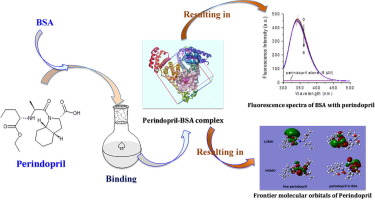
The binding characteristics of BSA with perindopril, a third-generation angiotensin-converting enzyme inhibitor, was investigated using various spectroscopic approaches combined with molecular simulation including molecular docking and density functional theory calculation. Meantime, the influence of some trace metal ions on the binding characteristics of BSA with perindopril was investigated. The findings indicated the perindopril quenched BSA fluorescence in a static way. The values of binding site (n) and binding constant (Kb) of perindopril on BSA were close to 1 and 8.12×103 M-1 at 298 K, respectively, indicating that the binding affinity was moderate and the stoichiometric ratio of the perindopril-BSA complex was 1:1. Thermodynamic analysis results revealed that the ΔG0, ΔH0, and ΔS0 values were negative, demonstrate the binding interaction was a spontaneous and exothermic process, the interaction forces were mainly hydrogen bonds and van der Waals forces. The results of the replacement experiments and molecular simulations indicated that perindopril bound to the groove between sub-domain IIB and IIA. Furthermore, the results also revealed the BSA conformation altered slightly during binding with perindopril and the existence of transition metal ions would result in the increase in the Kb value of perindopril on BSA, resulting in the decline in the concentration of free perindopril. The frontier molecular orbitals and other quantum chemical parameters as well as conformation of perindopril in BSA complex obviously altered for forming the more stable perindopril-BSA complex.
中文翻译:

使用多光谱,分子对接和DFT计算探索牛血清白蛋白与培哚普利之间的结合相互作用以及金属离子的影响
使用各种光谱方法结合分子模拟,包括分子对接和密度泛函理论计算,研究了牛血清白蛋白与第三代血管紧张素转化酶抑制剂培哚普利的结合特性。同时,研究了一些微量金属离子对BSA与培哚普利结合特性的影响。该发现表明培哚普利以静态方式猝灭了BSA荧光。培哚普利在BSA上的结合位点(n)和结合常数(K b)值分别接近1和8.12×10 3 M -1。分别在298 K的温度下,表明结合亲和力适中,培哚普利-BSA复合物的化学计量比为1:1。热力学分析结果表明,ΔG 0,ΔH 0,和ΔS 0值分别为负,表明结合相互作用是自发和放热过程中,相互作用力主要为氢键和范德华力。置换实验和分子模拟的结果表明,培哚普利与亚结构域IIB和IIA之间的凹槽结合。此外,结果还显示,在与培哚普利结合期间,BSA构象稍有改变,过渡金属离子的存在将导致K b的增加。培哚普利对BSA的影响,导致游离培哚普利浓度降低。BSA络合物的前沿分子轨道和其他量子化学参数以及培哚普利的构象发生了明显变化,从而形成了更稳定的培哚普利-BSA络合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号