当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Balancing Physicochemical Properties of Phenylthiazole Compounds with Antibacterial Potency by Modifying the Lipophilic Side Chain.
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2019-11-27 , DOI: 10.1021/acsinfecdis.9b00211 Ahmed Mancy 1 , Nader S Abutaleb 2 , Mohamed M Elsebaei 1 , Abdullah Y Saad 1 , Ahmed Kotb 1 , Alsagher O Ali 2, 3 , Jelan A Abdel-Aleem 2, 4 , Haroon Mohammad 2 , Mohamed N Seleem 2, 5 , Abdelrahman S Mayhoub 1, 6
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2019-11-27 , DOI: 10.1021/acsinfecdis.9b00211 Ahmed Mancy 1 , Nader S Abutaleb 2 , Mohamed M Elsebaei 1 , Abdullah Y Saad 1 , Ahmed Kotb 1 , Alsagher O Ali 2, 3 , Jelan A Abdel-Aleem 2, 4 , Haroon Mohammad 2 , Mohamed N Seleem 2, 5 , Abdelrahman S Mayhoub 1, 6
Affiliation
|
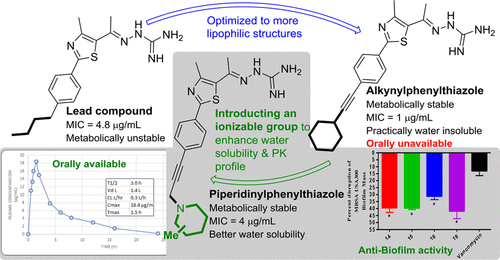
Bacterial resistance to antibiotics is presently one of the most pressing healthcare challenges and necessitates the discovery of new antibacterials with unique chemical scaffolds. However, the determination of the optimal balance between structural requirements for pharmacological action and pharmacokinetic properties of novel antibacterial compounds is a significant challenge in drug development. The incorporation of lipophilic moieties within a compound's core structure can enhance biological activity but have a deleterious effect on drug-like properties. In this Article, the lipophilicity of alkynylphenylthiazoles, previously identified as novel antibacterial agents, was reduced by introducing cyclic amines to the lipophilic side chain. In this regard, substitution with methylpiperidine (compounds 14-16) and thiomorpholine (compound 19) substituents significantly enhanced the aqueous solubility profile of the new compounds more than 150-fold compared to the first-generation lead compound 1b. Consequently, the pharmacokinetic profile of compound 15 was significantly enhanced with a notable improvement in both half-life and the time the compound's plasma concentration remained above its minimum inhibitory concentration (MIC) against methicillin-resistant Staphylococcus aureus (MRSA). In addition, compounds 14-16 and 19 were found to exert a bactericidal mode of action against MRSA and were not susceptible to resistance formation after 14 serial passages. Moreover, these compounds (at 2× MIC) were superior to the antibiotic vancomycin in the disruption of the mature MRSA biofilm. The modifications to the alkynylphenylthiazoles reported herein successfully improved the pharmacokinetic profile of this new series while maintaining the compounds' biological activity against MRSA.
中文翻译:

通过修饰亲脂性侧链平衡具有抗菌效力的苯基噻唑化合物的理化性质。
细菌对抗生素的抗性目前是最紧迫的医疗保健挑战之一,因此有必要发现具有独特化学支架的新型抗菌剂。然而,确定新的抗菌化合物的药理作用的结构要求和药代动力学性质之间的最佳平衡是药物开发中的重大挑战。在化合物的核心结构中掺入亲脂性部分可以增强生物活性,但对类似药物的性质具有有害作用。在本文中,通过将环胺引入亲脂性侧链,降低了先前被确定为新型抗菌剂的炔基苯基噻唑的亲脂性。在这方面,与第一代先导化合物1b相比,用甲基哌啶(化合物14-16)和硫代吗啉(化合物19)取代基取代可显着提高新化合物的水溶解度150倍以上。因此,化合物15的药代动力学曲线得到显着增强,同时半衰期和化合物血浆浓度保持高于其对耐甲氧西林金黄色葡萄球菌(MRSA)的最小抑菌浓度(MIC)以上的时间均得到显着改善。另外,发现化合物14-16和19对MRSA具有杀菌作用,并且在连续14次传代后不易形成抗性。此外,这些化合物(在2x MIC下)在破坏成熟的MRSA生物膜方面优于抗生素万古霉素。
更新日期:2019-11-28
中文翻译:

通过修饰亲脂性侧链平衡具有抗菌效力的苯基噻唑化合物的理化性质。
细菌对抗生素的抗性目前是最紧迫的医疗保健挑战之一,因此有必要发现具有独特化学支架的新型抗菌剂。然而,确定新的抗菌化合物的药理作用的结构要求和药代动力学性质之间的最佳平衡是药物开发中的重大挑战。在化合物的核心结构中掺入亲脂性部分可以增强生物活性,但对类似药物的性质具有有害作用。在本文中,通过将环胺引入亲脂性侧链,降低了先前被确定为新型抗菌剂的炔基苯基噻唑的亲脂性。在这方面,与第一代先导化合物1b相比,用甲基哌啶(化合物14-16)和硫代吗啉(化合物19)取代基取代可显着提高新化合物的水溶解度150倍以上。因此,化合物15的药代动力学曲线得到显着增强,同时半衰期和化合物血浆浓度保持高于其对耐甲氧西林金黄色葡萄球菌(MRSA)的最小抑菌浓度(MIC)以上的时间均得到显着改善。另外,发现化合物14-16和19对MRSA具有杀菌作用,并且在连续14次传代后不易形成抗性。此外,这些化合物(在2x MIC下)在破坏成熟的MRSA生物膜方面优于抗生素万古霉素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号