Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
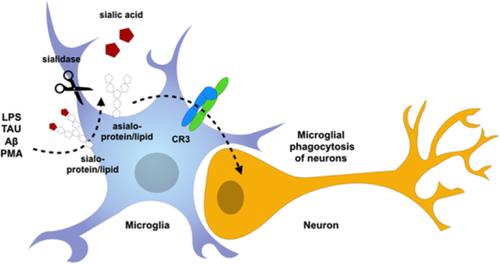
Activated microglia desialylate their surface, stimulating complement receptor 3-mediated phagocytosis of neurons.
Glia ( IF 5.4 ) Pub Date : 2019-11-27 , DOI: 10.1002/glia.23757 David H Allendorf 1 , Mar Puigdellívol 1 , Guy C Brown 1
Glia ( IF 5.4 ) Pub Date : 2019-11-27 , DOI: 10.1002/glia.23757 David H Allendorf 1 , Mar Puigdellívol 1 , Guy C Brown 1
Affiliation
|
The glycoproteins and glycolipids of the cell surface have sugar chains that normally terminate in a sialic acid residue, but inflammatory activation of myeloid cells can cause sialidase enzymes to remove these residues, resulting in desialylation and altered activity of surface receptors, such as the phagocytic complement receptor 3 (CR3). We found that activation of microglia with lipopolysaccharide (LPS), fibrillar amyloid beta (Aβ), Tau or phorbol myristate acetate resulted in increased surface sialidase activity and desialylation of the microglial surface. Desialylation of microglia by adding sialidase, stimulated microglial phagocytosis of beads, but this was prevented by siRNA knockdown of CD11b or a blocking antibody to CD11b (a component of CR3). Desialylation of microglia by a sialyl-transferase inhibitor (3FAx-peracetyl-Neu5Ac) also stimulated microglial phagocytosis of beads. Desialylation of primary glial-neuronal co-cultures by adding sialidase or the sialyl-transferase inhibitor resulted in neuronal loss that was prevented by inhibiting phagocytosis with cytochalasin D or the blocking antibody to CD11b. Adding desialylated microglia to glial-neuronal cultures, in the absence of neuronal desialylation, also caused neuronal loss prevented by CD11b blocking antibody. Adding LPS or Aβ to primary glial-neuronal co-cultures caused neuronal loss, and this was prevented by inhibiting endogenous sialidase activity with N-acetyl-2,3-dehydro-2-deoxyneuraminic acid or blockage of CD11b. Thus, activated microglia release a sialidase activity that desialylates the cell surface, stimulating CR3-mediated phagocytosis of neurons, making extracellular sialidase and CR3 potential treatment targets to prevent inflammatory loss of neurons.
中文翻译:

活化的小胶质细胞脱唾液酸化它们的表面,刺激补体受体3介导的神经元吞噬作用。
细胞表面的糖蛋白和糖脂具有通常在唾液酸残基中终止的糖链,但是髓样细胞的炎性活化可导致唾液酸酶去除这些残基,导致脱唾液酸化和表面受体(例如吞噬补体)的活性改变受体3(CR3)。我们发现,小胶质细胞与脂多糖(LPS),纤维状淀粉样蛋白β(Aβ),Tau或佛波醇肉豆蔻酸酯的活化导致表面唾液酸酶活性增加和小胶质细胞表面脱唾液酸化。通过添加唾液酸酶使小胶质细胞脱唾液酸化,刺激小珠的小胶质细胞吞噬作用,但这可通过siRNA敲低CD11b或CD11b的阻断抗体(CR3的成分)来防止。唾液酸转移酶抑制剂(3FAx-过乙酰基-Neu5Ac)对小胶质细胞的去唾液酸化作用也刺激了小胶质细胞吞噬小珠。通过添加唾液酸酶或唾液酸转移酶抑制剂对原代神经胶质-神经元共培养物进行去唾液酸化,可导致神经元损失,而这种损失可通过抑制细胞松弛素D或CD11b的阻断抗体来抑制吞噬作用来预防。在不存在神经元脱唾液酸化作用的情况下,向神经胶质神经元培养物中添加去唾液酸化的小胶质细胞,也会引起CD11b阻断抗体阻止的神经元丢失。向原代神经胶质-神经元共培养物中添加LPS或Aβ会引起神经元丢失,这可以通过用N-乙酰基-2,3-脱氢-2-脱氧神经氨酸抑制内源性唾液酸酶活性或阻断CD11b来防止。因此,活化的小胶质细胞释放出唾液酸酶活性,从而使细胞表面脱唾液酸化,
更新日期:2019-11-28
中文翻译:

活化的小胶质细胞脱唾液酸化它们的表面,刺激补体受体3介导的神经元吞噬作用。
细胞表面的糖蛋白和糖脂具有通常在唾液酸残基中终止的糖链,但是髓样细胞的炎性活化可导致唾液酸酶去除这些残基,导致脱唾液酸化和表面受体(例如吞噬补体)的活性改变受体3(CR3)。我们发现,小胶质细胞与脂多糖(LPS),纤维状淀粉样蛋白β(Aβ),Tau或佛波醇肉豆蔻酸酯的活化导致表面唾液酸酶活性增加和小胶质细胞表面脱唾液酸化。通过添加唾液酸酶使小胶质细胞脱唾液酸化,刺激小珠的小胶质细胞吞噬作用,但这可通过siRNA敲低CD11b或CD11b的阻断抗体(CR3的成分)来防止。唾液酸转移酶抑制剂(3FAx-过乙酰基-Neu5Ac)对小胶质细胞的去唾液酸化作用也刺激了小胶质细胞吞噬小珠。通过添加唾液酸酶或唾液酸转移酶抑制剂对原代神经胶质-神经元共培养物进行去唾液酸化,可导致神经元损失,而这种损失可通过抑制细胞松弛素D或CD11b的阻断抗体来抑制吞噬作用来预防。在不存在神经元脱唾液酸化作用的情况下,向神经胶质神经元培养物中添加去唾液酸化的小胶质细胞,也会引起CD11b阻断抗体阻止的神经元丢失。向原代神经胶质-神经元共培养物中添加LPS或Aβ会引起神经元丢失,这可以通过用N-乙酰基-2,3-脱氢-2-脱氧神经氨酸抑制内源性唾液酸酶活性或阻断CD11b来防止。因此,活化的小胶质细胞释放出唾液酸酶活性,从而使细胞表面脱唾液酸化,











































 京公网安备 11010802027423号
京公网安备 11010802027423号