当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Optimized Inhibitors of MDM2 via an Attempted Protein-Templated Reductive Amination.
ChemMedChem ( IF 3.6 ) Pub Date : 2019-12-12 , DOI: 10.1002/cmdc.201900574 Ramon van der Vlag 1 , M Yagiz Unver 1 , Tommaso Felicetti 1, 2 , Aleksandra Twarda-Clapa 3 , Fatima Kassim 1 , Cagdas Ermis 1 , Constantinos G Neochoritis 4, 5 , Bogdan Musielak 3 , Beata Labuzek 3 , Alexander Dömling 4 , Tad A Holak 3 , Anna K H Hirsch 1, 6, 7
ChemMedChem ( IF 3.6 ) Pub Date : 2019-12-12 , DOI: 10.1002/cmdc.201900574 Ramon van der Vlag 1 , M Yagiz Unver 1 , Tommaso Felicetti 1, 2 , Aleksandra Twarda-Clapa 3 , Fatima Kassim 1 , Cagdas Ermis 1 , Constantinos G Neochoritis 4, 5 , Bogdan Musielak 3 , Beata Labuzek 3 , Alexander Dömling 4 , Tad A Holak 3 , Anna K H Hirsch 1, 6, 7
Affiliation
|
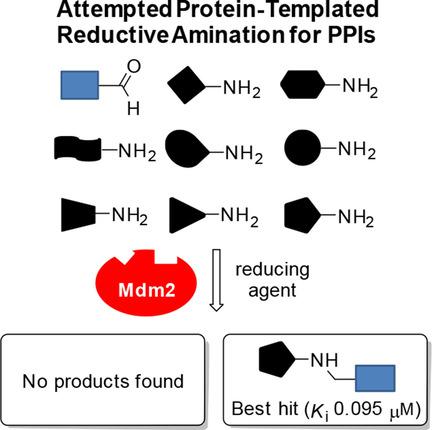
Innovative and efficient hit-identification techniques are required to accelerate drug discovery. Protein-templated fragment ligations represent a promising strategy in early drug discovery, enabling the target to assemble and select its binders from a pool of building blocks. Development of new protein-templated reactions to access a larger structural diversity and expansion of the variety of targets to demonstrate the scope of the technique are of prime interest for medicinal chemists. Herein, we present our attempts to use a protein-templated reductive amination to target protein-protein interactions (PPIs), a challenging class of drug targets. We address a flexible pocket, which is difficult to achieve by structure-based drug design. After careful analysis we did not find one of the possible products in the kinetic target-guided synthesis (KTGS) approach, however subsequent synthesis and biochemical evaluation of each library member demonstrated that all the obtained molecules inhibit MDM2. The most potent library member (Ki =0.095 μm) identified is almost as active as Nutlin-3, a potent inhibitor of the p53-MDM2 PPI.
中文翻译:

通过尝试的蛋白质模板还原胺化优化 MDM2 抑制剂。
需要创新和高效的命中识别技术来加速药物发现。蛋白质模板片段连接代表了早期药物发现中一种有前途的策略,使靶标能够从构建块池中组装和选择其结合物。药物化学家最感兴趣的是开发新的蛋白质模板反应以获得更大的结构多样性并扩展靶标的多样性以证明该技术的范围。在此,我们提出了使用蛋白质模板还原胺化来靶向蛋白质-蛋白质相互作用(PPI)的尝试,这是一类具有挑战性的药物靶点。我们解决了灵活的口袋问题,这很难通过基于结构的药物设计来实现。经过仔细分析,我们没有在动力学靶标引导合成(KTGS)方法中找到任何一种可能的产物,但随后对每个库成员的合成和生化评估表明,所有获得的分子都抑制 MDM2。鉴定出的最有效的文库成员 (Ki =0.095 μm) 几乎与 Nutlin-3 一样活跃,Nutlin-3 是 p53-MDM2 PPI 的有效抑制剂。
更新日期:2019-12-13
中文翻译:

通过尝试的蛋白质模板还原胺化优化 MDM2 抑制剂。
需要创新和高效的命中识别技术来加速药物发现。蛋白质模板片段连接代表了早期药物发现中一种有前途的策略,使靶标能够从构建块池中组装和选择其结合物。药物化学家最感兴趣的是开发新的蛋白质模板反应以获得更大的结构多样性并扩展靶标的多样性以证明该技术的范围。在此,我们提出了使用蛋白质模板还原胺化来靶向蛋白质-蛋白质相互作用(PPI)的尝试,这是一类具有挑战性的药物靶点。我们解决了灵活的口袋问题,这很难通过基于结构的药物设计来实现。经过仔细分析,我们没有在动力学靶标引导合成(KTGS)方法中找到任何一种可能的产物,但随后对每个库成员的合成和生化评估表明,所有获得的分子都抑制 MDM2。鉴定出的最有效的文库成员 (Ki =0.095 μm) 几乎与 Nutlin-3 一样活跃,Nutlin-3 是 p53-MDM2 PPI 的有效抑制剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号