当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Repurposing of FDA-Approved Drugs for Treating Iatrogenic Botulism: A Paired 3D-QSAR/Docking Approach†.
ChemMedChem ( IF 3.6 ) Pub Date : 2019-11-27 , DOI: 10.1002/cmdc.201900594 Giuseppe Floresta 1 , Vincenzo Patamia 1 , Davide Gentile 1 , Franco Molteni 2 , Andrea Santamato 3 , Antonio Rescifina 1, 4 , Michele Vecchio 5
ChemMedChem ( IF 3.6 ) Pub Date : 2019-11-27 , DOI: 10.1002/cmdc.201900594 Giuseppe Floresta 1 , Vincenzo Patamia 1 , Davide Gentile 1 , Franco Molteni 2 , Andrea Santamato 3 , Antonio Rescifina 1, 4 , Michele Vecchio 5
Affiliation
|
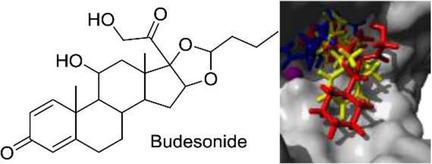
Botulinum neurotoxin (BoNT) is widely used for the treatment of spasticity, focal dystonia, chronic migraine, facial hemispasm, and facial aesthetic treatments. Generally, treatment with botulinum toxin is a safe procedure when conducted by clinicians with expertise, and local side effects are rare and transient. However, occasionally adverse effects can occur due to the spread of the drug to nontargeted muscles and organs, producing dry mouth, fatigue, and flu-like symptoms, up to signs of systemic botulism, which appears to be more frequent in children treated for spasticity than in adults. In silico 3D-QSAR and molecular docking studies were performed to build a structure-based model on selected potent known botulinum neurotoxin type A inhibitors; this was used to screen the US Food and Drug Administration (FDA) database. Thirty molecules were identified as possible light-chain BoNT/A inhibitors. In this study, we applied a well-established ligand- and structure-based methodology for the identification of hit compounds among a database of FDA-approved drugs. The identification of budesonide, protirelin, and ciclesonide followed by other compounds can be considered a starting point for investigations of selected compounds that could bypass much of the time and costs involved in the drug approval process.
中文翻译:

重新使用FDA批准的治疗医源性肉毒杆菌中毒的药物:配对的3D-QSAR /对接方法†。
肉毒杆菌神经毒素(BoNT)被广泛用于治疗痉挛,局灶性肌张力障碍,慢性偏头痛,面部偏瘫和面部美容治疗。通常,由具有专业知识的临床医生进行肉毒杆菌毒素治疗是一种安全的程序,而且局部副作用很少且是短暂的。但是,由于药物扩散到非针对性的肌肉和器官,产生口干,疲劳和类似流感的症状,有时甚至会出现不良反应,甚至出现全身性肉毒中毒的迹象,这种情况在接受痉挛治疗的儿童中似乎更为常见比成年人 在计算机上进行了3D-QSAR和分子对接研究,以在选定的已知有效A型肉毒杆菌神经毒素抑制剂上建立基于结构的模型;用来筛选美国食品和药物管理局(FDA)的数据库。已鉴定出三十种分子可能是轻链BoNT / A抑制剂。在这项研究中,我们应用了完善的基于配体和结构的方法来鉴定FDA批准的药物数据库中的命中化合物。鉴定布地奈德,普瑞瑞林和克索奈德后再鉴定其他化合物,可以视为研究所选化合物的起点,这些化合物可能会绕开药品批准过程中涉及的大量时间和成本。
更新日期:2019-12-09
中文翻译:

重新使用FDA批准的治疗医源性肉毒杆菌中毒的药物:配对的3D-QSAR /对接方法†。
肉毒杆菌神经毒素(BoNT)被广泛用于治疗痉挛,局灶性肌张力障碍,慢性偏头痛,面部偏瘫和面部美容治疗。通常,由具有专业知识的临床医生进行肉毒杆菌毒素治疗是一种安全的程序,而且局部副作用很少且是短暂的。但是,由于药物扩散到非针对性的肌肉和器官,产生口干,疲劳和类似流感的症状,有时甚至会出现不良反应,甚至出现全身性肉毒中毒的迹象,这种情况在接受痉挛治疗的儿童中似乎更为常见比成年人 在计算机上进行了3D-QSAR和分子对接研究,以在选定的已知有效A型肉毒杆菌神经毒素抑制剂上建立基于结构的模型;用来筛选美国食品和药物管理局(FDA)的数据库。已鉴定出三十种分子可能是轻链BoNT / A抑制剂。在这项研究中,我们应用了完善的基于配体和结构的方法来鉴定FDA批准的药物数据库中的命中化合物。鉴定布地奈德,普瑞瑞林和克索奈德后再鉴定其他化合物,可以视为研究所选化合物的起点,这些化合物可能会绕开药品批准过程中涉及的大量时间和成本。











































 京公网安备 11010802027423号
京公网安备 11010802027423号